Human CD8 T cells generated in vitro from hematopoietic stem cells are functionally mature
- PMID: 21429219
- PMCID: PMC3072939
- DOI: 10.1186/1471-2172-12-22
Human CD8 T cells generated in vitro from hematopoietic stem cells are functionally mature
Abstract
Background: T cell development occurs within the highly specialized thymus. Cytotoxic CD8 T cells are critical in adaptive immunity by targeting virally infected or tumor cells. In this study, we addressed whether functional CD8 T cells can be generated fully in vitro using human umbilical cord blood (UCB) hematopoietic stem cells (HSCs) in coculture with OP9-DL1 cells.
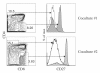
Results: HSC/OP9-DL1 cocultures supported the differentiation of CD8 T cells, which were TCR/CD3(hi) CD27(hi) CD1a(neg) and thus phenotypically resembled mature functional CD8 single positive thymocytes. These in vitro-generated T cells also appeared to be conventional CD8 cells, as they expressed high levels of Eomes and low levels of Plzf, albeit not identical to ex vivo UCB CD8 T cells. Consistent with the phenotypic and molecular characterization, upon TCR-stimulation, in vitro-generated CD8 T cells proliferated, expressed activation markers (MHC-II, CD25, CD38), secreted IFN-γ and expressed Granzyme B, a cytotoxic T-cell effector molecule.
Conclusion: Taken together, the ability to direct human hematopoietic stem cell or T-progenitor cells towards a mature functional phenotype raises the possibility of establishing cell-based treatments for T-immunodeficiencies by rapidly restoring CD8 effector function, thereby mitigating the risks associated with opportunistic infections.
Figures
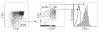




References
-
- Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, Berenson JR, Collins RH, Koup RA. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355:1875–1881. doi: 10.1016/S0140-6736(00)02293-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

