Tegument protein control of latent herpesvirus establishment and animation
- PMID: 21429246
- PMCID: PMC3063196
- DOI: 10.1186/2042-4280-2-3
Tegument protein control of latent herpesvirus establishment and animation
Abstract
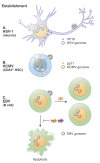
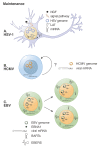
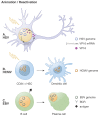
Herpesviruses are successful pathogens that infect most vertebrates as well as at least one invertebrate species. Six of the eight human herpesviruses are widely distributed in the population. Herpesviral infections persist for the life of the infected host due in large part to the ability of these viruses to enter a non-productive, latent state in which viral gene expression is limited and immune detection and clearance is avoided. Periodically, the virus will reactivate and enter the lytic cycle, producing progeny virus that can spread within or to new hosts. Latency has been classically divided into establishment, maintenance, and reactivation phases. Here we focus on demonstrated and postulated molecular mechanisms leading to the establishment of latency for representative members of each human herpesvirus family. Maintenance and reactivation are also briefly discussed. In particular, the roles that tegument proteins may play during latency are highlighted. Finally, we introduce the term animation to describe the initiation of lytic phase gene expression from a latent herpesvirus genome, and discuss why this step should be separated, both molecularly and theoretically, from reactivation.
Figures
Similar articles
-
Role of Epitranscriptomic and Epigenetic Modifications during the Lytic and Latent Phases of Herpesvirus Infections.Microorganisms. 2022 Aug 30;10(9):1754. doi: 10.3390/microorganisms10091754. Microorganisms. 2022. PMID: 36144356 Free PMC article. Review.
-
Herpesviral Latency-Common Themes.Pathogens. 2020 Feb 15;9(2):125. doi: 10.3390/pathogens9020125. Pathogens. 2020. PMID: 32075270 Free PMC article. Review.
-
Multifaceted Roles of ICP22/ORF63 Proteins in the Life Cycle of Human Herpesviruses.Front Microbiol. 2021 Jun 7;12:668461. doi: 10.3389/fmicb.2021.668461. eCollection 2021. Front Microbiol. 2021. PMID: 34163446 Free PMC article. Review.
-
A human herpesvirus 6A-encoded microRNA: role in viral lytic replication.J Virol. 2015 Mar;89(5):2615-27. doi: 10.1128/JVI.02007-14. Epub 2014 Dec 17. J Virol. 2015. PMID: 25520507 Free PMC article.
-
Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo.J Virol. 2006 Nov;80(22):10919-30. doi: 10.1128/JVI.01253-06. Epub 2006 Aug 30. J Virol. 2006. PMID: 16943285 Free PMC article.
Cited by
-
The gammaherpesvirus 68 viral cyclin facilitates expression of LANA.PLoS Pathog. 2021 Nov 15;17(11):e1010019. doi: 10.1371/journal.ppat.1010019. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34780571 Free PMC article.
-
Neuronal Stress Pathway Mediating a Histone Methyl/Phospho Switch Is Required for Herpes Simplex Virus Reactivation.Cell Host Microbe. 2015 Dec 9;18(6):649-58. doi: 10.1016/j.chom.2015.11.007. Cell Host Microbe. 2015. PMID: 26651941 Free PMC article.
-
Expanding the Known Functional Repertoire of the Human Cytomegalovirus pp71 Protein.Front Cell Infect Microbiol. 2020 Mar 12;10:95. doi: 10.3389/fcimb.2020.00095. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32226778 Free PMC article. Review.
-
Alpha- and gammaherpesviruses in stranded striped dolphins (Stenella coeruleoalba) from Spain: first molecular detection of gammaherpesvirus infection in central nervous system of odontocetes.BMC Vet Res. 2020 Aug 12;16(1):288. doi: 10.1186/s12917-020-02511-3. BMC Vet Res. 2020. PMID: 32787898 Free PMC article.
-
First description of a lesion in the upper digestive mucosa associated with a novel gammaherpesvirus in a striped dolphin (Stenella coeruleoalba) stranded in the Western Mediterranean Sea.BMC Vet Res. 2023 Aug 10;19(1):118. doi: 10.1186/s12917-023-03677-2. BMC Vet Res. 2023. PMID: 37563731 Free PMC article.
References
-
- Pellet P, Roizman B. In: Fields Virology. 5. Howley P, editor. Philadelphia: Lippincott; 2007. Herpesviridae: A Brief Introduction; pp. 2480–2499.
LinkOut - more resources
Full Text Sources

