Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction
- PMID: 21429478
- PMCID: PMC3090477
- DOI: 10.1016/j.biopsych.2011.01.023
Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction
Abstract
Background: Cue reactivity, the ability of cues associated with addictive substances to induce seeking and withdrawal, is a major contributor to addiction. Although human imaging studies show that cigarette-associated cues simultaneously activate the insula and the orbitofrontal cortex and evoke craving, how these activities functionally contribute to distinct elements of cue reactivity remains unclear. Moreover, it remains unclear whether the simultaneous activation of these cortical regions reflects coordinated functional connectivity or parallel processing.
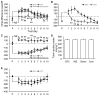
Methods: We selectively lesioned the insula or orbitofrontal cortex with the excitotoxin ibotenic acid in mice, and their approach to nicotine-associated cues (n = 6-13/group) and avoidance of withdrawal-associated cues (n = 5-12/group) were separately examined in place conditioning paradigms. We additionally tested the role of these two cortical structures in approach to food-associated cues (n = 6-7/group) and avoidance of lithium chloride-associated cues (n = 6-7/group).
Results: Our data show a double dissociation in which excitotoxic lesions of the insula and orbitofrontal cortex selectively disrupted nicotine-induced cue approach and withdrawal-induced cue avoidance, respectively. These effects were not entirely generalized to approach to food-associated cues or avoidance of lithium chloride-associated cues.
Conclusions: Our data provide functional evidence that cue reactivity seen in addiction includes unique neuroanatomically dissociable elements and suggest that the simultaneous activation of these two cortical regions in response to smoking-related cues does not necessarily indicate functional connectivity.
Copyright © 2011 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Craving and Cue Reactivity in Nicotine-Dependent Tobacco Smokers Is Associated With Different Insula Networks.Biol Psychiatry Cogn Neurosci Neuroimaging. 2020 Jan;5(1):76-83. doi: 10.1016/j.bpsc.2019.09.005. Epub 2019 Sep 23. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020. PMID: 31706906 Free PMC article.
-
Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers.Addict Biol. 2015 Mar;20(2):407-14. doi: 10.1111/adb.12124. Epub 2014 Feb 13. Addict Biol. 2015. PMID: 24529072 Free PMC article.
-
Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery.Psychopharmacology (Berl). 2006 Mar;184(3-4):577-88. doi: 10.1007/s00213-005-0080-x. Epub 2005 Aug 13. Psychopharmacology (Berl). 2006. PMID: 16133128
-
Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies.Neurosci Biobehav Rev. 2014 Jan;38:1-16. doi: 10.1016/j.neubiorev.2013.10.013. Epub 2013 Nov 6. Neurosci Biobehav Rev. 2014. PMID: 24211373 Free PMC article. Review.
-
Cue reactivity in nicotine and tobacco dependence: a "multiple-action" model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli.Brain Res Brain Res Rev. 2005 Feb;48(1):74-97. doi: 10.1016/j.brainresrev.2004.08.005. Brain Res Brain Res Rev. 2005. PMID: 15708629 Review.
Cited by
-
Temporal Dynamics of Large-Scale Networks Predict Neural Cue Reactivity and Cue-Induced Craving.Biol Psychiatry Cogn Neurosci Neuroimaging. 2020 Nov;5(11):1011-1018. doi: 10.1016/j.bpsc.2020.07.006. Epub 2020 Jul 18. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020. PMID: 32900658 Free PMC article.
-
Anterior insula stimulation suppresses appetitive behavior while inducing forebrain activation in alcohol-preferring rats.Transl Psychiatry. 2020 May 18;10(1):150. doi: 10.1038/s41398-020-0833-7. Transl Psychiatry. 2020. PMID: 32424183 Free PMC article.
-
Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors.Neuropsychopharmacology. 2013 Mar;38(4):690-8. doi: 10.1038/npp.2012.235. Epub 2012 Nov 15. Neuropsychopharmacology. 2013. PMID: 23249816 Free PMC article.
-
Cigarette exposure, dependence, and craving are related to insula thickness in young adult smokers.Neuropsychopharmacology. 2014 Jul;39(8):1816-22. doi: 10.1038/npp.2014.48. Epub 2014 Mar 3. Neuropsychopharmacology. 2014. PMID: 24584328 Free PMC article.
-
Continuous High Frequency Deep Brain Stimulation of the Rat Anterior Insula Attenuates the Relapse Post Withdrawal and Strengthens the Extinction of Morphine Seeking.Front Psychiatry. 2020 Oct 14;11:577155. doi: 10.3389/fpsyt.2020.577155. eCollection 2020. Front Psychiatry. 2020. PMID: 33173522 Free PMC article.
References
-
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. J Abnorm Psychol. 1988;97:133–152. - PubMed
-
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. - PubMed
-
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. - PubMed
-
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–687. - PubMed
-
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

