N-linked glycosylation of dengue virus NS1 protein modulates secretion, cell-surface expression, hexamer stability, and interactions with human complement
- PMID: 21429549
- PMCID: PMC3089955
- DOI: 10.1016/j.virol.2011.02.022
N-linked glycosylation of dengue virus NS1 protein modulates secretion, cell-surface expression, hexamer stability, and interactions with human complement
Abstract
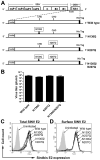
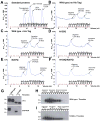
Dengue virus (DENV) NS1 is a versatile non-structural glycoprotein that is secreted as a hexamer, binds to the cell surface of infected and uninfected cells, and has immune evasive functions. DENV NS1 displays two conserved N-linked glycans at N130 and N207. In this study, we examined the role of these two N-linked glycans on NS1 secretion, stability, and function. Because some groups have reported reduced yields of infectious DENV when N130 and N207 are changed, we analyzed glycosylation-deficient NS1 phenotypes using a transgenic expression system. We show that the N-linked glycan at position 130 is required for stabilization of the secreted hexamer whereas the N-linked glycan at residue 207 facilitates secretion and extracellular protein stability. Moreover, NS1 mutants lacking an N-linked glycan at N130 did not interact efficiently with complement components C1s and C4. In summary, our results elucidate the contribution of N-linked glycosylation to the function of DENV NS1.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Alcon-LePoder S, Drouet MT, Roux P, Frenkiel MP, Arborio M, Durand-Schneider AM, Maurice M, Le Blanc I, Gruenberg J, Flamand M. The secreted form of dengue virus nonstructural protein NS1 is endocytosed by hepatocytes and accumulates in late endosomes: implications for viral infectivity. J Virol. 2005;79(17):11403–11. - PMC - PubMed
-
- Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40(2):376–81. - PMC - PubMed
-
- Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, Jairungsri A, Kanlaya R, Tangthawornchaikul N, Puttikhunt C, Pattanakitsakul SN, Yenchitsomanus PT, Mongkolsapaya J, Kasinrerk W, Sittisombut N, Husmann M, Blettner M, Vasanawathana S, Bhakdi S, Malasit P. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis. 2006;193(8):1078–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

