The in vitro fidelity of yeast DNA polymerase δ and polymerase ε holoenzymes during dinucleotide microsatellite DNA synthesis
- PMID: 21429821
- PMCID: PMC3121764
- DOI: 10.1016/j.dnarep.2011.02.003
The in vitro fidelity of yeast DNA polymerase δ and polymerase ε holoenzymes during dinucleotide microsatellite DNA synthesis
Abstract
Elucidating the sources of genetic variation within microsatellite alleles has important implications for understanding the etiology of human diseases. Mismatch repair is a well described pathway for the suppression of microsatellite instability. However, the cellular polymerases responsible for generating microsatellite errors have not been fully described. We address this gap in knowledge by measuring the fidelity of recombinant yeast polymerase δ (Pol δ) and ɛ (Pol ɛ) holoenzymes during synthesis of a [GT/CA] microsatellite. The in vitro HSV-tk forward assay was used to measure DNA polymerase errors generated during gap-filling of complementary GT(10) and CA(10)-containing substrates and ∼90 nucleotides of HSV-tk coding sequence surrounding the microsatellites. The observed mutant frequencies within the microsatellites were 4 to 30-fold higher than the observed mutant frequencies within the coding sequence. More specifically, the rate of Pol δ and Pol ɛ misalignment-based insertion/deletion errors within the microsatellites was ∼1000-fold higher than the rate of insertion/deletion errors within the HSV-tk gene. Although the most common microsatellite error was the deletion of a single repeat unit, ∼ 20% of errors were deletions of two or more units for both polymerases. The differences in fidelity for wild type enzymes and their exonuclease-deficient derivatives were ∼2-fold for unit-based microsatellite insertion/deletion errors. Interestingly, the exonucleases preferentially removed potentially stabilizing interruption errors within the microsatellites. Since Pol δ and Pol ɛ perform not only the bulk of DNA replication in eukaryotic cells but also are implicated in performing DNA synthesis associated with repair and recombination, these results indicate that microsatellite errors may be introduced into the genome during multiple DNA metabolic pathways.
Copyright © 2011 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

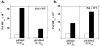

References
-
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435–445. - PubMed
-
- Hannan AJ. Tandem repeat polymorphisms: modulators of disease susceptibility and candidates for ‘missing heritability’. Trends Genet. 2010;26:59–65. - PubMed
-
- Chu CS, Trapnell BC, Curristin S, Cutting GR, Crystal RG. Genetic basis of variable exon 9 skipping in cystic fibrosis transmembrane conductance regulator mRNA. Nat Genet. 1993;3:151–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

