IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development
- PMID: 21429986
- PMCID: PMC3074453
- DOI: 10.1242/dev.054338
IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development
Abstract
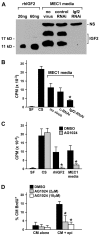
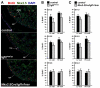
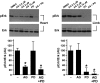
Secreted factors from the epicardium are believed to be important in directing heart ventricular cardiomyocyte proliferation and morphogenesis, although the specific factors involved have not been identified or characterized adequately. We found that IGF2 is the most prominent mitogen made by primary mouse embryonic epicardial cells and by a newly derived immortalized mouse embryonic epicardial cell line called MEC1. In vivo, Igf2 is expressed in the embryonic mouse epicardium during midgestation heart development. Using a whole embryo culture assay in the presence of inhibitors, we confirmed that IGF signaling is required to activate the ERK proliferation pathway in the developing heart, and that the epicardium is required for this response. Global disruption of the Igf2 gene, or conditional disruption of the two IGF receptor genes Igf1r and Insr together in the myocardium, each resulted in a significant decrease in ventricular wall proliferation and in ventricular wall hypoplasia. Ventricular cardiomyocyte proliferation in mutant embryos was restored to normal at E14.5, concurrent with the establishment of coronary circulation. Our results define IGF2 as a previously unexplored epicardial mitogen that is required for normal ventricular chamber development.
Figures



References
-
- Armstrong M. T., Lee D. Y., Armstrong P. B. (2000). Regulation of proliferation of the fetal myocardium. Dev. Dyn. 219, 226-236 - PubMed
-
- Austin A. F., Compton L. A., Love J. D., Brown C. B., Barnett J. V. (2008). Primary and immortalized mouse epicardial cells undergo differentiation in response to TGFbeta. Dev. Dyn. 237, 366-376 - PubMed
-
- Baker J., Liu J. P., Robertson E. J., Efstratiadis A. (1993). Role of insulin-like growth factors in embryonic and postnatal growth. Cell 75, 73-82 - PubMed
-
- Bondy C. A., Werner H., Roberts C. T., LeRoith D. (1990). Cellular pattern of insulin-like growth factor-I (IGF-I) and type I IGF receptor gene expression in early organogenesis: comparison with IGF-II gene expression. Mol. Endocrinol. 4, 1386-1398 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

