HGP44 induces protection against Porphyromonas gingivalis-Induced alveolar bone loss in mice
- PMID: 21430125
- PMCID: PMC3122540
- DOI: 10.1128/CVI.00556-10
HGP44 induces protection against Porphyromonas gingivalis-Induced alveolar bone loss in mice
Abstract
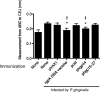
The protective effect of DNA vaccines expressing the Arg-gingipain A domain against bone loss induced by Porphyromonas gingivalis infection was investigated in a murine model. phgp44, which expresses the 44-kDa adhesion/hemagglutinin domain of Arg-gingipain A, prevented P. gingivalis-induced alveolar bone loss. The results indicate that phgp44 could be a candidate antigen for a vaccine against P. gingivalis infection.
Figures



References
-
- Amano A., Nakagawa I., Okahashi N., Hamada N. 2004. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal. Res. 39:136–142 - PubMed
-
- Baker P. J., Evans R. T., Roopenian D. C. 1994. Oral infection with Porphyromonas gingivalis and induced alveolar bone loss in immunocompetent and severe combined immunodeficient mice. Arch. Oral Biol. 39:1035–1040 - PubMed
-
- Darveau R. P. 2010. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 8:481–490 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

