Recovery from mu-opioid receptor desensitization after chronic treatment with morphine and methadone
- PMID: 21430144
- PMCID: PMC3092436
- DOI: 10.1523/JNEUROSCI.4874-10.2011
Recovery from mu-opioid receptor desensitization after chronic treatment with morphine and methadone
Abstract
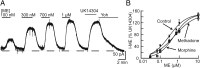
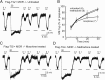
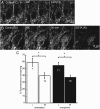
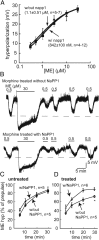
Chronic treatment with morphine results in a decrease in μ-opioid receptor sensitivity, an increase in acute desensitization, and a reduction in the recovery from acute desensitization in locus ceruleus neurons. With acute administration, morphine is unlike many other opioid agonists in that it does not mediate robust acute desensitization or induce receptor trafficking. This study compares μ-opioid receptor desensitization and trafficking in brain slices taken from rats treated for 6-7 d with a range of doses of morphine (60, 30, and 15 mg · kg(-1) · d(-1)) and methadone (60, 30, and 5 mg · kg(-1) · d(-1)) applied by subcutaneous implantation of osmotic minipumps. Mice were treated with 45 mg · kg(-1) · d(-1). In morphine-treated animals, recovery from acute [Met](5)enkephalin-induced desensitization and receptor recycling was diminished. In contrast, recovery and recycling were unchanged in slices from methadone-treated animals. Remarkably the reduced recovery from desensitization and receptor recycling found in slices from morphine-treated animals were not observed in animals lacking β-arrestin-2. Furthermore, pharmacological inhibition of G-protein receptor kinase 2 (GRK2), although not affecting the ability of [Met](5)enkephalin to induce desensitization, acutely reversed the delay in recovery from desensitization produced by chronic morphine treatment. These results characterize a previously unidentified function of the GRK/arrestin system in mediating opioid regulation in response to chronic morphine administration. They also suggest that the GRK/arrestin system, rather than serving as a primary mediator of acute desensitization, controls recovery from desensitization by regulating receptor reinsertion to the plasma membrane after chronic treatment with morphine. The sustained GRK/arrestin-dependent desensitization is another way in which morphine and methadone are distinguished.
Figures





Similar articles
-
Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons.Br J Pharmacol. 2012 Mar;165(6):1704-1716. doi: 10.1111/j.1476-5381.2011.01482.x. Br J Pharmacol. 2012. PMID: 21564086 Free PMC article. Review.
-
Cellular morphine tolerance produced by βarrestin-2-dependent impairment of μ-opioid receptor resensitization.J Neurosci. 2011 May 11;31(19):7122-30. doi: 10.1523/JNEUROSCI.5999-10.2011. J Neurosci. 2011. PMID: 21562274 Free PMC article.
-
Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons.J Neurosci. 2007 May 9;27(19):5092-104. doi: 10.1523/JNEUROSCI.1157-07.2007. J Neurosci. 2007. PMID: 17494695 Free PMC article.
-
Changes in the expression of G protein-coupled receptor kinases and beta-arrestin 2 in rat brain during opioid tolerance and supersensitivity.J Neurochem. 2001 Apr;77(2):486-92. doi: 10.1046/j.1471-4159.2001.00268.x. J Neurochem. 2001. PMID: 11299311
-
Remifentanil produces cross-desensitization and tolerance with morphine on the mu-opioid receptor.Neuropharmacology. 2013 Oct;73:368-79. doi: 10.1016/j.neuropharm.2013.06.010. Epub 2013 Jun 21. Neuropharmacology. 2013. PMID: 23792280
Cited by
-
β-Arrestin-2 knockout prevents development of cellular μ-opioid receptor tolerance but does not affect opioid-withdrawal-related adaptations in single PAG neurons.Br J Pharmacol. 2015 Jan;172(2):492-500. doi: 10.1111/bph.12673. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24597632 Free PMC article.
-
Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons.Br J Pharmacol. 2012 Mar;165(6):1704-1716. doi: 10.1111/j.1476-5381.2011.01482.x. Br J Pharmacol. 2012. PMID: 21564086 Free PMC article. Review.
-
Inhibition of β-ARK1 Ameliorates Morphine-induced Tolerance and Hyperalgesia Via Modulating the Activity of Spinal NMDA Receptors.Mol Neurobiol. 2018 Jun;55(6):5393-5407. doi: 10.1007/s12035-017-0780-3. Epub 2017 Oct 3. Mol Neurobiol. 2018. PMID: 28975565
-
Desensitization and Tolerance of Mu Opioid Receptors on Pontine Kölliker-Fuse Neurons.Mol Pharmacol. 2018 Jan;93(1):8-13. doi: 10.1124/mol.117.109603. Epub 2017 Nov 2. Mol Pharmacol. 2018. PMID: 29097440 Free PMC article.
-
Opioid Pharmacology under the Microscope.Mol Pharmacol. 2020 Oct;98(4):425-432. doi: 10.1124/mol.119.119321. Epub 2020 Mar 20. Mol Pharmacol. 2020. PMID: 32198210 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
