Input-specific critical periods for experience-dependent plasticity in layer 2/3 pyramidal neurons
- PMID: 21430146
- PMCID: PMC3066457
- DOI: 10.1523/JNEUROSCI.6042-10.2011
Input-specific critical periods for experience-dependent plasticity in layer 2/3 pyramidal neurons
Abstract
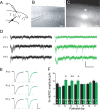
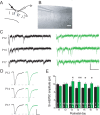
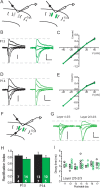
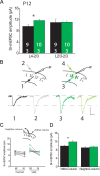
Critical periods for experience-dependent plasticity have been well characterized within sensory cortex, in which the ability of altered sensory input to drive firing rate changes has been demonstrated across brain areas. Here we show that rapid experience-dependent changes in the strength of excitatory synapses within mouse primary somatosensory cortex exhibit a critical period that is input specific and mechanistically distinct in layer 2/3 pyramidal neurons. Removal of all but a single whisker [single whisker experience (SWE)] can trigger the strengthening of individual glutamatergic synaptic contacts onto layer 2/3 neurons only during a short window during the second and third postnatal week. At both layer 4 and putative 2/3 inputs, SWE-triggered plasticity has a discrete onset, before which it cannot be induced. SWE synaptic strengthening is concluded at both inputs after the beginning of the third postnatal week, indicating that both types of inputs display a critical period for experience-dependent plasticity. Importantly, the timing of this critical period is both delayed and prolonged for layer 2/3-2/3 versus layer 4-2/3 excitatory synapses. Furthermore, plasticity at layer 2/3 inputs does not invoke the trafficking of calcium-permeable, GluR2-lacking AMPA receptors, whereas it sometimes does at layer 4 inputs. The dissociation of critical period timing and plasticity mechanisms at layer 4 and layer 2/3 synapses, despite the close apposition of these inputs along the dendrite, suggests remarkable specificity for the developmental regulation of plasticity in vivo.
Figures


Similar articles
-
Synaptic connections between layer 4 spiny neurone-layer 2/3 pyramidal cell pairs in juvenile rat barrel cortex: physiology and anatomy of interlaminar signalling within a cortical column.J Physiol. 2002 Feb 1;538(Pt 3):803-22. doi: 10.1113/jphysiol.2001.012959. J Physiol. 2002. PMID: 11826166 Free PMC article.
-
Timing-based LTP and LTD at vertical inputs to layer II/III pyramidal cells in rat barrel cortex.Neuron. 2000 Jul;27(1):45-56. doi: 10.1016/s0896-6273(00)00008-8. Neuron. 2000. PMID: 10939330
-
Potentiation of convergent synaptic inputs onto pyramidal neurons in somatosensory cortex: dependence on brain wave frequencies and NMDA receptor subunit composition.Neuroscience. 2014 Jul 11;272:271-85. doi: 10.1016/j.neuroscience.2014.04.062. Epub 2014 May 9. Neuroscience. 2014. PMID: 24814019
-
Developmental synaptic plasticity at the thalamocortical input to barrel cortex: mechanisms and roles.Mol Cell Neurosci. 2007 Apr;34(4):493-502. doi: 10.1016/j.mcn.2007.01.001. Epub 2007 Jan 10. Mol Cell Neurosci. 2007. PMID: 17329121 Free PMC article. Review.
-
Experience-dependent plasticity mechanisms for neural rehabilitation in somatosensory cortex.Philos Trans R Soc Lond B Biol Sci. 2009 Feb 12;364(1515):369-81. doi: 10.1098/rstb.2008.0252. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 19038777 Free PMC article. Review.
Cited by
-
Stimulus intensity determines experience-dependent modifications in neocortical neuron firing rates.Eur J Neurosci. 2015 Feb;41(4):410-9. doi: 10.1111/ejn.12805. Epub 2014 Dec 26. Eur J Neurosci. 2015. PMID: 25546174 Free PMC article.
-
Sensory input drives rapid homeostatic scaling of the axon initial segment in mouse barrel cortex.Nat Commun. 2021 Jan 4;12(1):23. doi: 10.1038/s41467-020-20232-x. Nat Commun. 2021. PMID: 33397944 Free PMC article.
-
Molecular states underlying neuronal cell type development and plasticity in the whisker cortex.bioRxiv [Preprint]. 2024 Oct 7:2024.10.07.617106. doi: 10.1101/2024.10.07.617106. bioRxiv. 2024. Update in: PLoS Biol. 2025 May 14;23(5):e3003176. doi: 10.1371/journal.pbio.3003176. PMID: 39416021 Free PMC article. Updated. Preprint.
-
Development and critical period plasticity of the barrel cortex.Eur J Neurosci. 2012 May;35(10):1540-53. doi: 10.1111/j.1460-9568.2012.08075.x. Eur J Neurosci. 2012. PMID: 22607000 Free PMC article. Review.
-
Initiation, labile, and stabilization phases of experience-dependent plasticity at neocortical synapses.J Neurosci. 2013 May 8;33(19):8483-93. doi: 10.1523/JNEUROSCI.3575-12.2013. J Neurosci. 2013. PMID: 23658185 Free PMC article.
References
-
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
