In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border
- PMID: 21430157
- PMCID: PMC3069132
- DOI: 10.1523/JNEUROSCI.4638-10.2011
In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border
Abstract
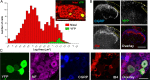
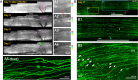
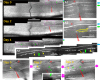
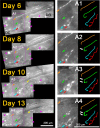
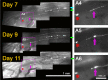
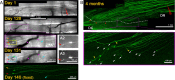
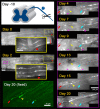
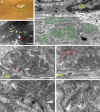
Dorsal root (DR) axons regenerate in the PNS but turn around or stop at the dorsal root entry zone (DREZ), the entrance into the CNS. Earlier studies that relied on conventional tracing techniques or postmortem analyses attributed the regeneration failure to growth inhibitors and lack of intrinsic growth potential. Here, we report the first in vivo imaging study of DR regeneration. Fluorescently labeled, large-diameter DR axons in thy1-YFPH mice elongated through a DR crush site, but not a transection site, and grew along the root at >1.5 mm/d with little variability. Surprisingly, they rarely turned around at the DREZ upon encountering astrocytes, but penetrated deeper into the CNS territory, where they rapidly stalled and then remained completely immobile or stable, even after conditioning lesions that enhanced growth along the root. Stalled axon tips and adjacent shafts were intensely immunolabeled with synapse markers. Ultrastructural analysis targeted to the DREZ enriched with recently arrived axons additionally revealed abundant axonal profiles exhibiting presynaptic features such as synaptic vesicles aggregated at active zones, but not postsynaptic features. These data suggest that axons are neither repelled nor continuously inhibited at the DREZ by growth-inhibitory molecules but are rapidly stabilized as they invade the CNS territory of the DREZ, forming presynaptic terminal endings on non-neuronal cells. Our work introduces a new experimental paradigm to the investigation of DR regeneration and may help to induce significant regeneration after spinal root injuries.
Figures



References
-
- Adcock KH, Brown DJ, Shearer MC, Shewan D, Schachner M, Smith GM, Geller HM, Fawcett JW. Axon behaviour at Schwann cell–astrocyte boundaries: manipulation of axon signalling pathways and the neural adhesion molecule L1 can enable axons to cross. Eur J Neurosci. 2004;20:1425–1435. - PubMed
-
- Allen NJ, Barres BA. Neuroscience: glia—more than just brain glue. Nature. 2009;457:675–677. - PubMed
-
- Beggah AT, Dours-Zimmermann MT, Barras FM, Brosius A, Zimmermann DR, Zurn AD. Lesion-induced differential expression and cell association of Neurocan, Brevican, Versican V1 and V2 in the mouse dorsal root entry zone. Neuroscience. 2005;133:749–762. - PubMed
-
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
