Analysis of the role of IL-21 in development of murine B cell progenitors in the bone marrow
- PMID: 21430229
- PMCID: PMC3080450
- DOI: 10.4049/jimmunol.1004040
Analysis of the role of IL-21 in development of murine B cell progenitors in the bone marrow
Abstract
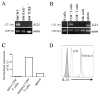
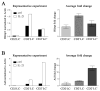
IL-21 plays a key role in the late stage of B cell development, where it has been shown to induce growth and differentiation of mature B cells into Ig-secreting plasma cells. Because IL-21R has also been reported on bone marrow (BM) B cell progenitors, we investigated whether IL-21R influenced earlier stages of B cell development. IL-21R is functional as early as the pro-B cell stage, and the strength of receptor-mediated signaling increases as cells mature. The addition of IL-21 to B cell progenitors in cell culture resulted in the accelerated appearance of mature B cell markers and was associated with the induction of Aid, Blimp1, and germline transcripts. We also found that stimulation of both IL-21R and CD40 was sufficient to induce the maturation of early B cell progenitors into IgM- and IgG-secreting cells. Consistent with a role for IL-21 in promoting B cell differentiation, the number of B220(+)CD43(+)IgM(-) pro-B cells was increased, and the number of mature IgM(hi)IgD(hi) cells was decreased in BM of IL-21R-deficient mice. We also report in this paper that IL-21 is expressed by BM CD4(+) T cells. These results provide evidence that IL-21R is functional in B cell progenitors and indicate that IL-21 regulates B cell development.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures





Similar articles
-
Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen.Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):2879-84. doi: 10.1073/pnas.1019764108. Epub 2011 Jan 31. Proc Natl Acad Sci U S A. 2011. PMID: 21282663 Free PMC article.
-
Combined signaling through interleukin-7 receptors and flt3 but not c-kit potently and selectively promotes B-cell commitment and differentiation from uncommitted murine bone marrow progenitor cells.Blood. 1996 Aug 15;88(4):1256-65. Blood. 1996. PMID: 8695843
-
IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow.J Immunol. 2009 Apr 1;182(7):4255-66. doi: 10.4049/jimmunol.0800489. J Immunol. 2009. PMID: 19299724 Free PMC article.
-
Liver sinusoidal endothelial cells promote B lymphopoiesis from primitive hematopoietic cells.Stem Cells Dev. 2010 Mar;19(3):341-50. doi: 10.1089/scd.2009.0300. Stem Cells Dev. 2010. PMID: 19788396
-
Bone marrow microenvironmental changes in aged mice compromise V(D)J recombinase activity and B cell generation.Semin Immunol. 2005 Oct;17(5):347-55. doi: 10.1016/j.smim.2005.05.012. Semin Immunol. 2005. PMID: 15963731 Review.
Cited by
-
The aged microenvironment impairs BCL6 and CD40L induction in CD4+ T follicular helper cell differentiation.Aging Cell. 2024 Jun;23(6):e14140. doi: 10.1111/acel.14140. Epub 2024 Mar 13. Aging Cell. 2024. PMID: 38481058 Free PMC article.
-
Gm40600 promotes CD4+ T-cell responses by interacting with Ahnak.Immunology. 2021 Sep;164(1):190-206. doi: 10.1111/imm.13365. Epub 2021 Jun 2. Immunology. 2021. PMID: 33987830 Free PMC article.
-
Extension of the germinal center stage of B cell development promotes autoantibodies in BXD2 mice.Arthritis Rheum. 2013 Oct;65(10):2703-12. doi: 10.1002/art.38059. Arthritis Rheum. 2013. PMID: 23818250 Free PMC article.
-
Per2 induction limits lymphoid-biased haematopoietic stem cells and lymphopoiesis in the context of DNA damage and ageing.Nat Cell Biol. 2016 May;18(5):480-90. doi: 10.1038/ncb3342. Epub 2016 Apr 18. Nat Cell Biol. 2016. PMID: 27088856
-
Hrd1-mediated BLIMP-1 ubiquitination promotes dendritic cell MHCII expression for CD4 T cell priming during inflammation.J Exp Med. 2014 Nov 17;211(12):2467-79. doi: 10.1084/jem.20140283. Epub 2014 Nov 3. J Exp Med. 2014. PMID: 25366967 Free PMC article.
References
-
- Lu L, Chaudhury P, Osmond DG. Regulation of cell survival during B lymphopoiesis: apoptosis and Bcl-2/Bax content of precursor B cells in bone marrow of mice with altered expression of IL-7 and recombinase-activating gene-2. J Immunol. 1999;162:1931–1940. - PubMed
-
- Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15:521–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

