Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids
- PMID: 21430254
- PMCID: PMC3077888
- DOI: 10.3945/jn.111.139485
Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids
Abstract
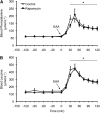
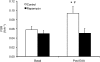
The relationship between mammalian target of rapamycin complex 1 (mTORC1) signaling and muscle protein synthesis during instances of amino acid surplus in humans is based solely on correlational data. Therefore, the goal of this study was to use a mechanistic approach specifically designed to determine whether increased mTORC1 activation is requisite for the stimulation of muscle protein synthesis following L-essential amino acid (EAA) ingestion in humans. Examination of muscle protein synthesis and signaling were performed on vastus lateralis muscle biopsies obtained from 8 young (25 ± 2 y) individuals who were studied prior to and following ingestion of 10 g of EAA during 2 separate trials in a randomized, counterbalanced design. The trials were identical except during 1 trial, participants were administered a single oral dose of a potent mTORC1 inhibitor (rapamycin) prior to EAA ingestion. In response to EAA ingestion, an ~60% increase in muscle protein synthesis was observed during the control trial, concomitant with increased phosphorylation of mTOR (Ser(2448)), ribosomal S6 kinase 1 (Thr(389)), and eukaryotic initiation factor 4E binding protein 1 (Thr(37/46)). In contrast, prior administration of rapamycin completely blocked the increase in muscle protein synthesis and blocked or attenuated activation of mTORC1-signaling proteins. The inhibition of muscle protein synthesis and signaling was not due to differences in either extracellular or intracellular amino acid availability, because these variables were similar between trials. These data support a fundamental role for mTORC1 activation as a key regulator of human muscle protein synthesis in response to increased EAA availability. This information will be useful in the development of evidence-based nutritional therapies targeting mTORC1 to counteract muscle wasting associated with numerous clinical conditions.
Trial registration: ClinicalTrials.gov NCT00891696.
Conflict of interest statement
Author disclosures: J. M. Dickinson, C. S. Fry, M. J. Drummond, D. M. Gundermann, D. K. Walker, E. L. Glynn, K. L. Timmerman, S. Dhanani, E. Volpi, and B. B. Rasmussen, no conflicts of interest.
Figures


References
-
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–8 - PubMed
-
- Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–400 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

