Colocalization of gadolinium-diethylene triamine pentaacetic acid with high-molecular-weight molecules after intracerebral convection-enhanced delivery in humans
- PMID: 21430586
- PMCID: PMC3154565
- DOI: 10.1227/NEU.0b013e3182181ba8
Colocalization of gadolinium-diethylene triamine pentaacetic acid with high-molecular-weight molecules after intracerebral convection-enhanced delivery in humans
Abstract
Background: Convection-enhanced delivery (CED) permits site-specific therapeutic drug delivery within interstitial spaces at increased dosages through circumvention of the blood-brain barrier. CED is currently limited by suboptimal methodologies for monitoring the delivery of therapeutic agents that would permit technical optimization and enhanced therapeutic efficacy.
Objective: To determine whether a readily available small-molecule MRI contrast agent, gadolinium-diethylene triamine pentaacetic acid (Gd-DTPA), could effectively track the distribution of larger therapeutic agents.
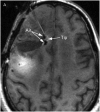
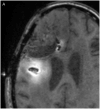
Methods: Gd-DTPA was coinfused with the larger molecular tracer, I-labeled human serum albumin (I-HSA), during CED of an EGFRvIII-specific immunotoxin as part of treatment for a patient with glioblastoma.
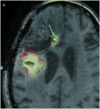
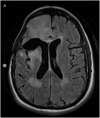
Results: Infusion of both tracers was safe in this patient. Analysis of both Gd-DTPA and I-HSA during and after infusion revealed a high degree of anatomical and volumetric overlap.
Conclusion: Gd-DTPA may be able to accurately demonstrate the anatomic and volumetric distribution of large molecules used for antitumor therapy with high resolution and in combination with fluid-attenuated inversion recovery (FLAIR) imaging, and provide additional information about leaks into cerebrospinal fluid spaces and resection cavities. Similar studies should be performed in additional patients to validate our findings and help refine the methodologies we used.
Figures














Similar articles
-
Convection-enhanced delivery of free gadolinium with the recombinant immunotoxin MR1-1.J Neurooncol. 2010 May;98(1):1-7. doi: 10.1007/s11060-009-0046-7. Epub 2009 Nov 7. J Neurooncol. 2010. PMID: 19898744 Free PMC article. Clinical Trial.
-
Magnetic Resonance Imaging-Based Assessment of Gadolinium-Conjugated Diethylenetriamine Penta-Acetic Acid Test-Infusion in Detecting Dysfunction of Convection-Enhanced Delivery Catheters.World Neurosurg. 2016 May;89:272-9. doi: 10.1016/j.wneu.2016.02.003. Epub 2016 Feb 6. World Neurosurg. 2016. PMID: 26862025 Clinical Trial.
-
Using MRI to Determine Drug Concentration in Convection-Enhanced Delivery: A Proof-of-Concept study.Neurosci Lett. 2025 Mar 10;851:138170. doi: 10.1016/j.neulet.2025.138170. Epub 2025 Feb 19. Neurosci Lett. 2025. PMID: 39983860
-
Imaging of convection enhanced delivery of toxins in humans.Toxins (Basel). 2011 Mar;3(3):201-6. doi: 10.3390/toxins3030201. Epub 2011 Mar 15. Toxins (Basel). 2011. PMID: 22069706 Free PMC article. Review.
-
Imaging of Convective Drug Delivery in the Nervous System.Neurosurg Clin N Am. 2017 Oct;28(4):615-622. doi: 10.1016/j.nec.2017.05.012. Epub 2017 Aug 19. Neurosurg Clin N Am. 2017. PMID: 28917289 Free PMC article. Review.
Cited by
-
Gene-based therapy of Parkinson's Disease: Translation from animal model to human clinical trial employing convection enhanced delivery.Ann Neurosci. 2012 Jul;19(3):133-46. doi: 10.5214/ans.0972.7531.190310. Ann Neurosci. 2012. PMID: 25205986 Free PMC article. Review.
-
Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier.Curr Pharm Des. 2016;22(9):1177-1193. doi: 10.2174/1381612822666151221150733. Curr Pharm Des. 2016. PMID: 26685681 Free PMC article. Review.
-
Novel delivery strategies for glioblastoma.Cancer J. 2012 Jan-Feb;18(1):89-99. doi: 10.1097/PPO.0b013e318244d8ae. Cancer J. 2012. PMID: 22290262 Free PMC article. Review.
-
Current status of intratumoral therapy for glioblastoma.J Neurooncol. 2015 Oct;125(1):1-7. doi: 10.1007/s11060-015-1875-1. Epub 2015 Aug 2. J Neurooncol. 2015. PMID: 26233248
-
Convection-enhanced delivery for high-grade glioma.Neurooncol Pract. 2021 Nov 20;9(1):24-34. doi: 10.1093/nop/npab065. eCollection 2022 Feb. Neurooncol Pract. 2021. PMID: 35096401 Free PMC article. Review.
References
-
- Grossi PM, Ochiai H, Archer GE, et al. Efficacy of intracerebral microinfusion of trastuzumab in an athymic rat model of intracerebral metastatic breast cancer. Clin Cancer Res. 2003 Nov 15;9(15):5514–5520. - PubMed
-
- Heimberger AB, Archer GE, McLendon RE, et al. Temozolomide delivered by intracerebral microinfusion is safe and efficacious against malignant gliomas in rats. Clin Cancer Res. 2000 Oct;6(10):4148–4153. - PubMed
-
- Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003 May;9(5):589–595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

