Opportunities for use of human iPS cells in predictive toxicology
- PMID: 21430658
- PMCID: PMC3593635
- DOI: 10.1038/clpt.2011.9
Opportunities for use of human iPS cells in predictive toxicology
Abstract
Toxicity assessment is a major challenge for cost-effective drug development, and there is great need for better tools to accurately predict adverse drug reactions. Technological advances are empowering new cell-based assays for predictive toxicology, and these assays are critically dependent on the cell source. Here we describe the properties of human induced pluripotent stem (iPS) cells that make them a promising cell source for toxicity assessment and highlight progress to date and important roadblocks remaining.
Conflict of interest statement
CONFLICT OF INTEREST / DISCLOSURES
TJK is a co-founder and consultant for Cellular Dynamics International which is a company that performs toxicity testing with cellular products derived from human iPS cells.
Figures

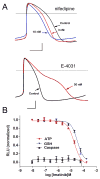
References
-
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–5. - PubMed
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. - PubMed
-
- Applications of Toxicogenomic Technologies to Predictive Toxicology and Risk Assessment. The National Academies Press; Washington, D.C: 2007. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

