Design, synthesis and biological characterization of novel inhibitors of CD38
- PMID: 21431168
- PMCID: PMC3357394
- DOI: 10.1039/c0ob00768d
Design, synthesis and biological characterization of novel inhibitors of CD38
Abstract
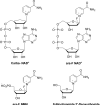
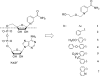
Human CD38 is a novel multi-functional protein that acts not only as an antigen for B-lymphocyte activation, but also as an enzyme catalyzing the synthesis of a Ca(2+) messenger molecule, cyclic ADP-ribose, from NAD(+). It is well established that this novel Ca(2+) signaling enzyme is responsible for regulating a wide range of physiological functions. Based on the crystal structure of the CD38/NAD(+) complex, we synthesized a series of simplified N-substituted nicotinamide derivatives (Compound 1-14). A number of these compounds exhibited moderate inhibition of the NAD(+) utilizing activity of CD38, with Compound 4 showing the highest potency. The crystal structure of CD38/Compound 4 complex and computer simulation of Compound 7 docking to CD38 show a significant role of the nicotinamide moiety and the distal aromatic group of the compounds for substrate recognition by the active site of CD38. Biologically, we showed that both Compounds 4 and 7 effectively relaxed the agonist-induced contraction of muscle preparations from rats and guinea pigs. This study is a rational design of inhibitors for CD38 that exhibit important physiological effects, and can serve as a model for future drug development.
Figures


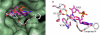
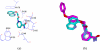



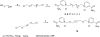

References
-
- Lee HC. Enzymatic functions and structures of CD38 and homologs. Chem. Immunol. 2000;75:39–59. - PubMed
-
- Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Ann. Rev. Pharmacol. Toxicol. 2001;41:317–345. - PubMed
- Lee HC, Aarhus R, Levitt D. The crystal structure of cyclic ADP-ribose. Nat. Struct. Biol. 1994;1:143–144. - PubMed
- Lee HC, Walseth TF, Bratt GT, et al. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 1989;264:1608–15. - PubMed
-
- Howard M, Grimaldi JC, Bazan JF, Lund FE, Santos-Argumedo L, Parkhouse RM, Walseth TF, Lee HC. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. - PubMed
-
- Kato I, Yamamoto Y, Fujimura M, Noguchi N, Takasawa S, Okamoto H. CD38 disruption impairs glucose-induced increases in Cyclic ADP-ribose, [Ca2+]i, and insulin secretion. J. Biol. Chem. 1999;274:1869–1872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

