Physiologically based pharmacokinetic model of amphotericin B disposition in rats following administration of deoxycholate formulation (Fungizone®): pooled analysis of published data
- PMID: 21431453
- PMCID: PMC3085707
- DOI: 10.1208/s12248-011-9267-8
Physiologically based pharmacokinetic model of amphotericin B disposition in rats following administration of deoxycholate formulation (Fungizone®): pooled analysis of published data
Abstract
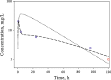
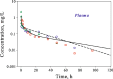
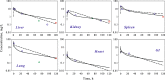
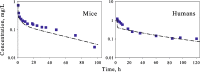
The time course of tissue distribution of amphotericin B (AmB) has not been sufficiently characterized despite its therapeutic importance and an apparent disconnect between plasma pharmacokinetics and clinical outcomes. The goals of this work were to develop and evaluate a physiologically based pharmacokinetic (PBPK) model to characterize the disposition properties of AmB administered as deoxycholate formulation in healthy rats and to examine the utility of the PBPK model for interspecies scaling of AmB pharmacokinetics. AmB plasma and tissue concentration-time data, following single and multiple intravenous administration of Fungizone® to rats, from several publications were combined for construction of the model. Physiological parameters were fixed to literature values. Various structural models for single organs were evaluated, and the whole-body PBPK model included liver, spleen, kidney, lung, heart, gastrointestinal tract, plasma, and remainder compartments. The final model resulted in a good simultaneous description of both single and multiple dose data sets. Incorporation of three subcompartments for spleen and kidney tissues was required for capturing a prolonged half-life in these organs. The predictive performance of the final PBPK model was assessed by evaluating its utility in predicting pharmacokinetics of AmB in mice and humans. Clearance and permeability-surface area terms were scaled with body weight. The model demonstrated good predictions of plasma AmB concentration-time profiles for both species. This modeling framework represents an important basis that may be further utilized for characterization of formulation- and disease-related factors in AmB pharmacokinetics and pharmacodynamics.
Figures



Similar articles
-
Biodistribution and histopathology studies of amphotericin B sodium deoxycholate sulfate formulation following intratracheal instillation in rat models.Drug Deliv Transl Res. 2020 Feb;10(1):59-69. doi: 10.1007/s13346-019-00662-x. Drug Deliv Transl Res. 2020. PMID: 31368043
-
Application of Physiologically Based Pharmacokinetic Model to Compare the Biodistribution of Liposomal Amphotericin B With Conventional Amphotericin B Deoxycholate in Humans.Biopharm Drug Dispos. 2024 Dec;45(4-6):208-219. doi: 10.1002/bdd.2406. Epub 2024 Dec 25. Biopharm Drug Dispos. 2024. PMID: 39722430
-
Dual physiologically based pharmacokinetic model of liposomal and nonliposomal amphotericin B disposition.Pharm Res. 2014 Jan;31(1):35-45. doi: 10.1007/s11095-013-1127-z. Epub 2013 Jun 21. Pharm Res. 2014. PMID: 23793994
-
Super aggregated form of Amphotericin B: a novel way to increase its therapeutic index.Curr Pharm Des. 2016;22(7):792-803. doi: 10.2174/1381612822666151209151719. Curr Pharm Des. 2016. PMID: 26648472 Review.
-
Continuous infusion of amphotericin B deoxycholate: an innovative, low-cost strategy in antifungal treatment.Mycoses. 2011 Mar;54(2):91-8. doi: 10.1111/j.1439-0507.2009.01805.x. Mycoses. 2011. PMID: 19878457 Review.
Cited by
-
Integration of In Vitro and In Vivo Models to Predict Cellular and Tissue Dosimetry of Nanomaterials Using Physiologically Based Pharmacokinetic Modeling.ACS Nano. 2022 Dec 27;16(12):19722-19754. doi: 10.1021/acsnano.2c07312. Epub 2022 Dec 15. ACS Nano. 2022. PMID: 36520546 Free PMC article. Review.
-
A Physiologically Based Pharmacokinetic Model of Amiodarone and its Metabolite Desethylamiodarone in Rats: Pooled Analysis of Published Data.Eur J Drug Metab Pharmacokinet. 2016 Dec;41(6):689-703. doi: 10.1007/s13318-015-0295-0. Eur J Drug Metab Pharmacokinet. 2016. PMID: 26254911
-
Streamlining physiologically-based pharmacokinetic model design for intravenous delivery of nanoparticle drugs.CPT Pharmacometrics Syst Pharmacol. 2022 Apr;11(4):409-424. doi: 10.1002/psp4.12762. Epub 2022 Feb 7. CPT Pharmacometrics Syst Pharmacol. 2022. PMID: 35045205 Free PMC article.
-
Application of pharmacokinetic and pharmacodynamic analysis to the development of liposomal formulations for oncology.Pharmaceutics. 2014 Mar 18;6(1):137-74. doi: 10.3390/pharmaceutics6010137. Pharmaceutics. 2014. PMID: 24647104 Free PMC article.
-
Short-Course Rather Than Low-Dose Amphotericin B May Exert Potential Influence on Mortality in Cryptococcal Meningitis Patients Treated With Amphotericin B Plus Flucytosine Alone or in Combination With Fluconazole.Front Microbiol. 2019 Sep 10;10:2082. doi: 10.3389/fmicb.2019.02082. eCollection 2019. Front Microbiol. 2019. PMID: 31551999 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

