Successful treatment of metastatic melanoma by adoptive transfer of blood-derived polyclonal tumor-specific CD4+ and CD8+ T cells in combination with low-dose interferon-alpha
- PMID: 21431917
- PMCID: PMC3119331
- DOI: 10.1007/s00262-011-1004-8
Successful treatment of metastatic melanoma by adoptive transfer of blood-derived polyclonal tumor-specific CD4+ and CD8+ T cells in combination with low-dose interferon-alpha
Abstract
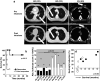
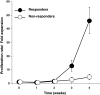
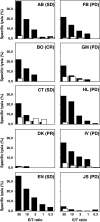
A phase I/II study was conducted to test the feasibility and safety of the adoptive transfer of tumor-reactive T cells and daily injections of interferon-alpha (IFNα) in metastatic melanoma patients with progressive disease. Autologous melanoma cell lines were established to generate tumor-specific T cells by autologous mixed lymphocyte tumor cell cultures using peripheral blood lymphocytes. Ten patients were treated with on average 259 (range 38-474) million T cells per infusion to a maximum of six infusions, and clinical response was evaluated according to the response evaluation criteria in solid tumors (RECIST). Five patients showed clinical benefit from this treatment, including one complete regression, one partial response, and three patients with stable disease. No treatment-related serious adverse events were observed, except for the appearance of necrotic-like fingertips in one patient. An IFNα-related transient leucopenia was detected in 6 patients, including all responders. One responding patient displayed vitiligo. The infused T-cell batches consisted of tumor-reactive polyclonal CD8+ and/or CD4+ T cells. Clinical reactivity correlated with the functional properties of the infused tumor-specific T cells, including their in vitro expansion rate and the secretion of mainly Th1 cytokines as opposed to Th2 cytokines. Our study shows that relatively low doses of T cells and low-dose IFNα can lead to successful treatment of metastatic melanoma and reveals a number of parameters potentially associated with this success.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma.J Clin Oncol. 2006 Nov 1;24(31):5060-9. doi: 10.1200/JCO.2006.07.1100. J Clin Oncol. 2006. PMID: 17075125 Clinical Trial.
-
IDO and galectin-3 hamper the ex vivo generation of clinical grade tumor-specific T cells for adoptive cell therapy in metastatic melanoma.Cancer Immunol Immunother. 2017 Jul;66(7):913-926. doi: 10.1007/s00262-017-1995-x. Epub 2017 Apr 11. Cancer Immunol Immunother. 2017. PMID: 28401257 Free PMC article.
-
Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients.Clin Cancer Res. 2012 Dec 15;18(24):6758-70. doi: 10.1158/1078-0432.CCR-12-1177. Epub 2012 Oct 2. Clin Cancer Res. 2012. PMID: 23032743 Free PMC article. Clinical Trial.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
-
White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy.Clin Cancer Res. 2011 Apr 1;17(7):1664-73. doi: 10.1158/1078-0432.CCR-10-2272. Epub 2011 Feb 15. Clin Cancer Res. 2011. PMID: 21325070 Review.
Cited by
-
Tumour-reactive B cells and antibody responses after allogeneic haematopoietic cell transplantation.Immunooncol Technol. 2020 Jul 23;7:15-22. doi: 10.1016/j.iotech.2020.07.002. eCollection 2020 Sep. Immunooncol Technol. 2020. PMID: 35754458 Free PMC article. Review.
-
Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids.Cell. 2018 Sep 6;174(6):1586-1598.e12. doi: 10.1016/j.cell.2018.07.009. Epub 2018 Aug 9. Cell. 2018. PMID: 30100188 Free PMC article.
-
Ex vivo enrichment of circulating anti-tumor T cells from both cutaneous and ocular melanoma patients: clinical implications for adoptive cell transfer therapy.Cancer Immunol Immunother. 2012 Aug;61(8):1169-82. doi: 10.1007/s00262-011-1179-z. Epub 2011 Dec 30. Cancer Immunol Immunother. 2012. PMID: 22207316 Free PMC article.
-
Enhanced antigen cross-presentation in human colorectal cancer-associated fibroblasts through upregulation of the lysosomal protease cathepsin S.J Immunother Cancer. 2022 Mar;10(3):e003591. doi: 10.1136/jitc-2021-003591. J Immunother Cancer. 2022. PMID: 35264435 Free PMC article.
-
Melanoma cells can be eliminated by sialylated CD43 × CD3 bispecific T cell engager formats in vitro and in vivo.Cancer Immunol Immunother. 2021 Jun;70(6):1569-1581. doi: 10.1007/s00262-020-02780-9. Epub 2020 Nov 23. Cancer Immunol Immunother. 2021. PMID: 33225419 Free PMC article.
References
-
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. - DOI - PMC - PubMed
-
- Posthuma EF, Marijt EW, Barge RM, van Soest RA, Baas IO, Starrenburg CW, van Zelderen-Bhola SL, Fibbe WE, Smit WM, Willemze R, Falkenburg JH. Alpha-interferon with very-low-dose donor lymphocyte infusion for hematologic or cytogenetic relapse of chronic myeloid leukemia induces rapid and durable complete remissions and is associated with acceptable graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10:204–212. doi: 10.1016/j.bbmt.2003.11.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

