DNA damage and estrogenic activity induced by the environmental pollutant 2-nitrotoluene and its metabolite
- PMID: 21432561
- PMCID: PMC2921039
- DOI: 10.1007/s12199-010-0146-1
DNA damage and estrogenic activity induced by the environmental pollutant 2-nitrotoluene and its metabolite
Abstract
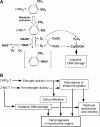
Objectives: The environmental pollutant 2-nitrotoluene (2-NO(2)-T) is carcinogenic and reproductively toxic in animals. In this study, we elucidated the mechanisms of its carcinogenicity and reproductive toxicity.
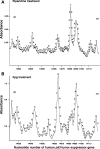
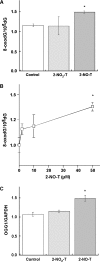
Methods: We examined DNA damage induced by 2-NO(2)-T and its metabolite, 2-nitrosotoluene (2-NO-T), using (32)P-5'-end-labeled DNA. We measured 8-oxo-7, 8-dihydro-2'-deoxyguanosine (8-oxodG), an indicator of oxidative DNA damage, in calf thymus DNA and cellular DNA in cultured human leukemia (HL-60) cells treated with 2-NO(2)-T and 2-NO-T. 8-Oxoguanine DNA glycosylase (OGG1) gene expression in HL-60 cells was measured by real-time polymerase chain reaction (PCR). We examined estrogenic activity using an E-screen assay and a surface plasmon resonance (SPR) sensor.
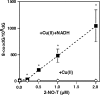
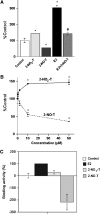
Results: In experiments with isolated DNA fragments, 2-NO-T induced oxidative DNA damage in the presence of Cu (II) and β-nicotinamide adenine dinucleotide disodium salt (reduced form) (NADH), while 2-NO(2)-T did not. 2-NO-T significantly increased levels of 8-oxodG in HL-60 cells. Real-time polymerase chain reaction (PCR) analysis revealed upregulation of OGG1 gene expression induced by 2-NO-T. An E-screen assay using the human breast cancer cell line MCF-7 revealed that 2-NO(2)-T induced estrogen-dependent cell proliferation. In contrast, 2-NO-T decreased the cell number and suppressed 17β-estradiol-induced cell proliferation. The data obtained with the SPR sensor using estrogen receptor α and the estrogen response element supported the results of the E-screen assay.
Conclusions: Oxidative DNA damage caused by 2-NO-T and estrogen-disrupting effects caused by 2-NO(2)-T and 2-NO-T may play a role in the reproductive toxicity and carcinogenicity of these entities.
Figures

Similar articles
-
Oxidative DNA damage induced by a melatonin metabolite, 6-hydroxymelatonin, via a unique non-o-quinone type of redox cycle.Biochem Pharmacol. 2004 Nov 1;68(9):1869-78. doi: 10.1016/j.bcp.2004.06.016. Biochem Pharmacol. 2004. PMID: 15450952
-
Oxidative DNA damage and mammary cell proliferation by alcohol-derived salsolinol.Chem Res Toxicol. 2013 Oct 21;26(10):1455-63. doi: 10.1021/tx400182n. Epub 2013 Sep 25. Chem Res Toxicol. 2013. PMID: 24020587
-
Genistein and daidzein induce cell proliferation and their metabolites cause oxidative DNA damage in relation to isoflavone-induced cancer of estrogen-sensitive organs.Biochemistry. 2004 Mar 9;43(9):2569-77. doi: 10.1021/bi035613d. Biochemistry. 2004. PMID: 14992594
-
Catechins induce oxidative damage to cellular and isolated DNA through the generation of reactive oxygen species.Free Radic Res. 2003 Aug;37(8):881-90. doi: 10.1080/1071576031000150751. Free Radic Res. 2003. PMID: 14567448
-
Cancer risk and oxidative DNA damage in man.J Mol Med (Berl). 1996 Jun;74(6):297-312. doi: 10.1007/BF00207507. J Mol Med (Berl). 1996. PMID: 8862511 Review.
Cited by
-
A Bph-Like Nitroarene Dioxygenase Catalyzes the Conversion of 3-Nitrotoluene to 3-Methylcatechol by Rhodococcus sp. Strain ZWL3NT.Appl Environ Microbiol. 2020 Feb 3;86(4):e02517-19. doi: 10.1128/AEM.02517-19. Print 2020 Feb 3. Appl Environ Microbiol. 2020. PMID: 31811044 Free PMC article.
-
Evaluation of estrogenic potential of flavonoids using a recombinant yeast strain and MCF7/BUS cell proliferation assay.PLoS One. 2013 Oct 1;8(10):e74881. doi: 10.1371/journal.pone.0074881. eCollection 2013. PLoS One. 2013. PMID: 24098354 Free PMC article.
-
Current insight into the transient X-zone in the adrenal gland cortex.Vitam Horm. 2024;124:297-339. doi: 10.1016/bs.vh.2023.05.003. Epub 2023 Jul 12. Vitam Horm. 2024. PMID: 38408801 Free PMC article. Review.
-
Assessment of the mode of action underlying development of rodent small intestinal tumors following oral exposure to hexavalent chromium and relevance to humans.Crit Rev Toxicol. 2013 Mar;43(3):244-74. doi: 10.3109/10408444.2013.768596. Crit Rev Toxicol. 2013. PMID: 23445218 Free PMC article. Review.
References
-
- Printing processes and printing inks, carbon black and some nitro compounds. Lyon: IARC; 1997.
-
- National Toxicology Program, National Institutes of Health, US Department of Health and Human Services. Toxicology and carcinogenesis studies of o-nitrotoluene sulfone (CAS no. 88-72-2) in F344/N rats and B6C3F(1) mice (feed studies), Natl Toxicol Program Tech Rep Ser. 2002;504:1–357. - PubMed
-
- National Toxicology Program, National Institutes of Health, US Department of Health and Human Services. Comparative toxicity and carcinogenicity studies of o-nitrotoluene and o-toluidine hydrochloride (CAS Nos. 88-72-2 and 636-21-5) administered in feed to male F344/N rats, Toxic Rep Ser. 2000;44:1–C8. - PubMed
-
- Hong HL, Ton TV, Devereux TR, Moomaw C, Clayton N, Chan P, Dunnick JK, Sills RC. Chemical-specific alterations in ras, p53, and beta-catenin genes in hemangiosarcomas from B6C3F1 mice exposed to o-nitrotoluene or riddelliine for 2 years. Toxicol Appl Pharmacol. 2003;191:227–234. doi: 10.1016/S0041-008X(03)00165-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
