MRI evaluation of axonal reorganization after bone marrow stromal cell treatment of traumatic brain injury
- PMID: 21432927
- PMCID: PMC3381889
- DOI: 10.1002/nbm.1667
MRI evaluation of axonal reorganization after bone marrow stromal cell treatment of traumatic brain injury
Abstract
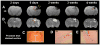
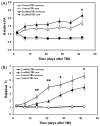
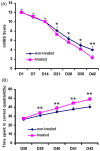
We treated traumatic brain injury (TBI) with human bone marrow stromal cells (hMSCs) and evaluated the effect of treatment on white matter reorganization using MRI. We subjected male Wistar rats (n = 17) to controlled cortical impact and either withheld treatment (controls; n = 9) or inserted collagen scaffolds containing hMSCs (n = 8). Six weeks later, the rats were sacrificed and MRI revealed selective migration of grafted neural progenitor cells towards the white matter reorganized boundary of the TBI-induced lesion. Histology confirmed that the white matter had been reorganized, associated with increased fractional anisotropy (FA; p < 0.01) in the recovery regions relative to the injured core region in both treated and control groups. Treatment with hMSCs increased FA in the recovery regions, lowered T(2) in the core region, decreased lesion volume and improved functional recovery relative to untreated controls. Immunoreactive staining showed axonal projections emanating from neurons and extruding from the corpus callosum into the ipsilateral cortex at the boundary of the lesion. Fiber tracking (FT) maps derived from diffusion tensor imaging confirmed the immunohistological data and provided information on axonal rewiring. The apparent kurtosis coefficient (AKC) detected additional axonal remodeling regions with crossing axons, confirmed by immunohistological staining, compared with FA. Our data demonstrate that AKC, FA, FT and T(2) can be used to evaluate treatment-induced white matter recovery, which may facilitate restorative therapy in patients with TBI.
Copyright © 2011 John Wiley & Sons, Ltd.
Figures


References
-
- Becker DP, Gade GF, Yound HF, Feuerman TF. Diagnosis and treatment of head injury in adults. In: Youmans JR, editor. Neurological Surgery. WB Saunders; Philadelphia, PA: 1990. pp. 2017–2148.
-
- Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19(5):503–557. - PMC - PubMed
-
- Dunnett SB, Bjorklund A, Lindvall O. Cell therapy in Parkinson’s disease – stop or go? Nat Rev Neurosci. 2001;2(5):365–369. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS43324/NS/NINDS NIH HHS/United States
- R01 NS048349/NS/NINDS NIH HHS/United States
- R01 NS38292/NS/NINDS NIH HHS/United States
- R01 HL064766/HL/NHLBI NIH HHS/United States
- R01 NS064134/NS/NINDS NIH HHS/United States
- P01 NS042345/NS/NINDS NIH HHS/United States
- P01 NS23393/NS/NINDS NIH HHS/United States
- R01 AG027852/AG/NIA NIH HHS/United States
- P01 NS023393/NS/NINDS NIH HHS/United States
- P50 NS023393/NS/NINDS NIH HHS/United States
- R01 NS038292/NS/NINDS NIH HHS/United States
- R01 NS42345/NS/NINDS NIH HHS/United States
- R01 HL64766/HL/NHLBI NIH HHS/United States
- R01 NS043324/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

