32-channel RF coil optimized for brain and cervical spinal cord at 3 T
- PMID: 21433068
- PMCID: PMC3131444
- DOI: 10.1002/mrm.22906
32-channel RF coil optimized for brain and cervical spinal cord at 3 T
Abstract
Diffusion and functional magnetic resonance imaging of the spinal cord remain challenging due to the small cross-sectional size of the cord and susceptibility-related distortions. Although partially addressable through parallel imaging, few highly parallel array coils have been implemented for the cervical cord. Here, we developed a 32-channel coil that fully covers the brain and c-spine and characterized its performance in comparison with a commercially available head/neck/spine array. Image and temporal signal-to-noise ratio were, respectively, increased by 2× and 1.8× in the cervical cord. Averaged g-factors at 4× acceleration were lowered by 22% in the brain and by 39% in the spinal cord, enabling 1-mm isotropic R = 4 multi-echo magnetization prepared gradient echo of the full brain and c-spine in 3:20 min. Diffusion imaging of the cord at 0.6 × 0.6 × 5 mm(3) resolution and tractography of the full brain and c-spine at 1.7-mm isotropic resolution were feasible without noticeable distortion. Improvements of this nature potentially enhance numerous basic and clinical research studies focused on spinal and supraspinal regions.
Copyright © 2011 Wiley-Liss, Inc.
Figures

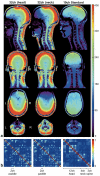


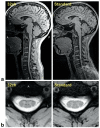
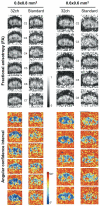
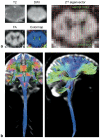
References
-
- Thurnher MM, Bammer R. Diffusion-weighted magnetic resonance imaging of the spine and spinal cord. Seminars in roentgenology. 2006;41(4):294–311. - PubMed
-
- Heidemann RM, Ozsarlak O, Parizel PM, Michiels J, Kiefer B, Jellus V, Müller M, Breuer F, Blaimer M, Griswold MA, Jakob PM. A brief review of parallel magnetic resonance imaging. Eur Radiol. 2003;13(10):2323–2337. - PubMed
-
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng H-M, Brady T, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89(12):5675–5679. - PMC - PubMed
-
- Giove F, Garreffa G, Giulietti G, Mangia S, Colonnese C, Maraviglia B. Issues about the fMRI of the human spinal cord. Magn Reson Imaging. 2004;22(10):1505–1516. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

