Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer
- PMID: 21433223
- PMCID: PMC3346716
- DOI: 10.1002/stem.632
Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer
Abstract
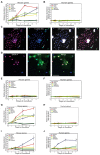
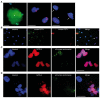
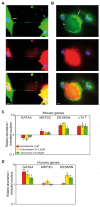
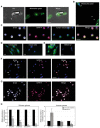
Because stem cells are often found to improve repair tissue including heart without evidence of engraftment or differentiation, mechanisms underlying wound healing are still elusive. Several studies have reported that stem cells can fuse with cardiomyocytes either by permanent or partial cell fusion processes. However, the respective physiological impact of these two processes remains unknown in part because of the lack of knowledge of the resulting hybrid cells. To further characterize cell fusion, we cocultured mouse fully differentiated cardiomyocytes with human multipotent adipose-derived stem (hMADS) cells as a model of adult stem cells. We found that heterologous cell fusion promoted cardiomyocyte reprogramming back to a progenitor-like state. The resulting hybrid cells expressed early cardiac commitment and proliferation markers such as GATA-4, myocyte enhancer factor 2C, Nkx2.5, and Ki67 and exhibited a mouse genotype. Interestingly, human bone marrow-derived stem cells shared similar reprogramming properties than hMADS cells but not human fibroblasts, which suggests that these features might be common to multipotent cells. Furthermore, cardiac hybrid cells were preferentially generated by partial rather than permanent cell fusion and that intercellular structures composed of f-actin and microtubule filaments were involved in the process. Finally, we showed that stem cell mitochondria were transferred into cardiomyocytes, persisted in hybrids and were required for somatic cell reprogramming. In conclusion, by providing new insights into previously reported cell fusion processes, our data might contribute to a better understanding of stem cell-mediated regenerative mechanisms and thus, the development of more efficient stem cell-based heart therapies.
Copyright © 2011 AlphaMed Press.
Conflict of interest statement
The authors declare they have no potential conflicts of interests.
Figures



Similar articles
-
Stem cell induced cardiac regeneration: fusion/mitochondrial exchange and/or transdifferentiation?Cell Cycle. 2011 Jul 15;10(14):2281-6. doi: 10.4161/cc.10.14.16513. Epub 2011 Jul 15. Cell Cycle. 2011. PMID: 21654195 Review.
-
Role of somatic cell sources in the maturation degree of human induced pluripotent stem cell-derived cardiomyocytes.Biochim Biophys Acta Mol Cell Res. 2020 Mar;1867(3):118538. doi: 10.1016/j.bbamcr.2019.118538. Epub 2019 Aug 28. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 31472168
-
Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation.Mol Ther. 2006 Dec;14(6):840-50. doi: 10.1016/j.ymthe.2006.05.016. Epub 2006 Sep 11. Mol Ther. 2006. PMID: 16965940
-
RNA-Based Strategies for Cardiac Reprogramming of Human Mesenchymal Stromal Cells.Cells. 2020 Feb 22;9(2):504. doi: 10.3390/cells9020504. Cells. 2020. PMID: 32098400 Free PMC article.
-
Recent advances in direct cardiac reprogramming.Curr Opin Genet Dev. 2015 Oct;34:77-81. doi: 10.1016/j.gde.2015.09.004. Epub 2015 Oct 24. Curr Opin Genet Dev. 2015. PMID: 26454285 Free PMC article. Review.
Cited by
-
Circulating around the tissue: hematopoietic cell-based fusion versus transdifferentiation.Circ Res. 2015 Feb 13;116(4):563-5. doi: 10.1161/CIRCRESAHA.115.305820. Circ Res. 2015. PMID: 25677514 Free PMC article. No abstract available.
-
Mitochondria: A Potential Rejuvenation Tool against Aging.Aging Dis. 2024 Apr 1;15(2):503-516. doi: 10.14336/AD.2023.0712. Aging Dis. 2024. PMID: 37815912 Free PMC article. Review.
-
Intercellular transport of Tau protein and β-amyloid mediated by tunneling nanotubes.Am J Transl Res. 2021 Nov 15;13(11):12509-12522. eCollection 2021. Am J Transl Res. 2021. PMID: 34956469 Free PMC article.
-
Connecting different heart diseases through intercellular communication.Biol Open. 2021 Sep 15;10(9):bio058777. doi: 10.1242/bio.058777. Epub 2021 Sep 8. Biol Open. 2021. PMID: 34494646 Free PMC article. Review.
-
Acute Myocardial Infarction Reduces Respiration in Rat Cardiac Fibers, despite Adipose Tissue Mesenchymal Stromal Cell Transplant.Stem Cells Int. 2020 Jun 20;2020:4327965. doi: 10.1155/2020/4327965. eCollection 2020. Stem Cells Int. 2020. PMID: 32655647 Free PMC article.
References
-
- D’Ippolito G, Diabira S, Howard GA, et al. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. - PubMed
-
- Reyes M, Lund T, Lenvik T, et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. - PubMed
-
- Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. - PubMed
-
- Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

