Quorum sensing between Pseudomonas aeruginosa biofilms accelerates cell growth
- PMID: 21434644
- PMCID: PMC3076519
- DOI: 10.1021/ja111131f
Quorum sensing between Pseudomonas aeruginosa biofilms accelerates cell growth
Abstract
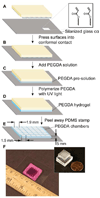
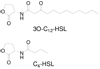
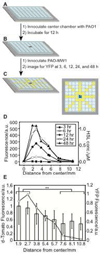
This manuscript describes the fabrication of arrays of spatially confined chambers embossed in a layer of poly(ethylene glycol) diacrylate (PEGDA) and their application to studying quorum sensing between communities of Pseudomonas aeruginosa. We hypothesized that biofilms may produce stable chemical signaling gradients in close proximity to surfaces, which influence the growth and development of nearby microcolonies into biofilms. To test this hypothesis, we embossed a layer of PEGDA with 1.5-mm wide chambers in which P. aeruginosa biofilms grew, secreted homoserine lactones (HSLs, small molecule regulators of quorum sensing), and formed spatial and temporal gradients of these compounds. In static growth conditions (i.e., no flow), nascent biofilms secreted N-(3-oxododecanoyl) HSL that formed a gradient in the hydrogel and was detected by P. aeruginosa cells that were ≤8 mm away. Diffusing HSLs increased the growth rate of cells in communities that were <3 mm away from the biofilm, where the concentration of HSL was >1 μM, and had little effect on communities farther away. The HSL gradient had no observable influence on biofilm structure. Surprisingly, 0.1-10 μM of N-(3-oxododecanoyl) HSL had no effect on cell growth in liquid culture. The results suggest that the secretion of HSLs from a biofilm enhances the growth of neighboring cells in contact with surfaces into communities and may influence their composition, organization, and diversity.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

