Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity
- PMID: 21434880
- PMCID: PMC3171869
- DOI: 10.1111/j.1476-5381.2011.01336.x
Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity
Abstract
Background and purpose: Up-regulation of thioredoxin interacting protein (TXNIP), an endogenous inhibitor of thioredoxin (Trx), compromises cellular antioxidant and anti-apoptotic defences and stimulates pro-inflammatory cytokines expression, implying a role for TXNIP in apoptosis. Here we have examined the causal role of TXNIP expression in mediating retinal neurotoxicity and assessed the neuroprotective actions of verapamil, a calcium channel blocker and an inhibitor of TXNIP expression.
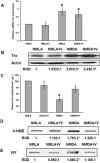
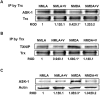
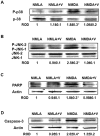
Experimental approach: Retinal neurotoxicity was induced by intravitreal injection of NMDA in Sprague-Dawley rats, which received verapamil (10 mg·kg(-1), p.o.) or vehicle. Neurotoxicity was examined by terminal dUTP nick-end labelling assay and ganglion cell count. Expression of TXNIP, apoptosis signal-regulating kinase 1 (ASK-1), NF-κB, p38 MAPK, JNK, cleaved poly-ADP-ribose polymerase (PARP), caspase-3, nitrotyrosine and 4-hydroxy-nonenal were examined by Western and slot-blot analysis. Release of TNF-α and IL-1β was examined by elisa.
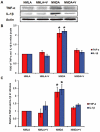
Key results: NMDA injection enhanced TXNIP expression, decreased Trx activity, causing increased oxidative stress, glial activation and release of TNF-α and IL-1β. Enhanced TXNIP expression disrupted Trx/ASK-1 inhibitory complex leading to release of ASK-1 and activation of the pro-apoptotic p38 MAPK/JNK pathway, as indicated by cleaved PARP and caspase-3 expression. Treatment with verapamil blocked these effects.
Conclusion and implications: Elevated TXNIP expression contributed to retinal neurotoxicity by three different mechanisms, inducing release of inflammatory mediators such as TNF-α and IL-1β, altering antioxidant status and disrupting the Trx-ASK-1 inhibitory complex leading to activation of the p38 MAPK/JNK apoptotic pathway. Targeting TXNIP expression is a potential therapeutic target for retinal neurodegenerative disease.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures




Similar articles
-
Deletion of thioredoxin-interacting protein preserves retinal neuronal function by preventing inflammation and vascular injury.Br J Pharmacol. 2014 Mar;171(5):1299-313. doi: 10.1111/bph.12535. Br J Pharmacol. 2014. PMID: 24283717 Free PMC article.
-
Neurovascular protective effect of FeTPPs in N-methyl-D-aspartate model: similarities to diabetes.Am J Pathol. 2010 Sep;177(3):1187-97. doi: 10.2353/ajpath.2010.091289. Epub 2010 Jul 22. Am J Pathol. 2010. PMID: 20651233 Free PMC article.
-
Thioredoxin-interacting protein is required for endothelial NLRP3 inflammasome activation and cell death in a rat model of high-fat diet.Diabetologia. 2014 Feb;57(2):413-23. doi: 10.1007/s00125-013-3101-z. Epub 2013 Nov 8. Diabetologia. 2014. PMID: 24201577 Free PMC article.
-
Exploring Thioredoxin-Interacting Protein (TXNIP) as a Therapeutic Target for Cardiovascular Diseases.Arch Pharm (Weinheim). 2025 Aug;358(8):e70082. doi: 10.1002/ardp.70082. Arch Pharm (Weinheim). 2025. PMID: 40851354 Review.
-
TXNIP: A Double-Edged Sword in Disease and Therapeutic Outlook.Oxid Med Cell Longev. 2022 Apr 11;2022:7805115. doi: 10.1155/2022/7805115. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35450411 Free PMC article. Review.
Cited by
-
Thioredoxin system regulation in the central nervous system: experimental models and clinical evidence.Oxid Med Cell Longev. 2014;2014:590808. doi: 10.1155/2014/590808. Epub 2014 Feb 27. Oxid Med Cell Longev. 2014. PMID: 24723994 Free PMC article. Review.
-
Verapamil as an Adjunct Therapy to Reduce tPA Toxicity in Hyperglycemic Stroke: Implication of TXNIP/NLRP3 Inflammasome.Mol Neurobiol. 2021 Aug;58(8):3792-3804. doi: 10.1007/s12035-021-02384-z. Epub 2021 Apr 13. Mol Neurobiol. 2021. PMID: 33847912 Free PMC article.
-
Deletion of thioredoxin interacting protein (TXNIP) augments hyperoxia-induced vaso-obliteration in a mouse model of oxygen induced-retinopathy.PLoS One. 2014 Oct 16;9(10):e110388. doi: 10.1371/journal.pone.0110388. eCollection 2014. PLoS One. 2014. PMID: 25329456 Free PMC article.
-
Gene therapy to treat inherited and complex retinal degenerative diseases.Mol Ther Methods Clin Dev. 2015 Aug 5;2:15027. doi: 10.1038/mtm.2015.27. eCollection 2015. Mol Ther Methods Clin Dev. 2015. PMID: 26251841 Free PMC article. No abstract available.
-
Role of inflammasome activation in the pathophysiology of vascular diseases of the neurovascular unit.Antioxid Redox Signal. 2015 May 1;22(13):1188-206. doi: 10.1089/ars.2014.6126. Epub 2014 Nov 11. Antioxid Redox Signal. 2015. PMID: 25275222 Free PMC article. Review.
References
-
- Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, El-Remessy AB. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther. 2010;332:125–134. - PubMed
-
- Ali TK, Matragoon S, Pillai BA, Liou GI, El-Remessy AB. Peroxynitrite mediates retinal neurodegeneration by inhibiting NGF survival signal in experimental and human diabetes. J Diabetes. 2008;57:889–898. - PubMed
-
- Ali TK, Al-Gayyar MMH, Matragoon S, Pillai BA, Abdelsaid MA, Nussbaum JJ, et al. Diabetes-induced peroxynitrite impairs the balance of ProNGF/NGF and causes neurovascular injury. Diabetologia. 2011;54:657–668. - PubMed
-
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

