Arvelexin from Brassica rapa suppresses NF-κB-regulated pro-inflammatory gene expression by inhibiting activation of IκB kinase
- PMID: 21434881
- PMCID: PMC3171867
- DOI: 10.1111/j.1476-5381.2011.01351.x
Arvelexin from Brassica rapa suppresses NF-κB-regulated pro-inflammatory gene expression by inhibiting activation of IκB kinase
Abstract
Background and purpose: Brassica rapa species constitute one of the major sources of food. In the present study, we investigated the anti-inflammatory effects and the underlying molecular mechanism of arvelexin, isolated from B. rapa, on lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages and on a model of septic shock induced by LPS.
Experimental approach: The expression of Inducible nitric oxide synthase (iNOS) and COX-2, TNF-α, IL-6 and IL-1β were determined by Western blot and/or RT-PCR respectively. To elucidate the underlying mechanism(s), activation of NF-κB activation and its pathways were investigated by electrophoretic mobility shift assay, reporter gene and Western blot assays. In addition, the in vivo anti-inflammatory effects of arvelexin were evaluated in endotoxaemia induced with LPS.
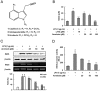
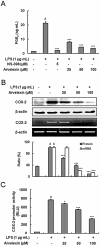
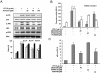
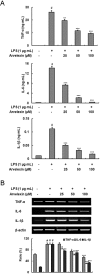
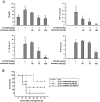
Key results: Promoter assays for iNOS and COX-2 revealed that arvelexin inhibited LPS-induced NO and prostaglandin E(2) production through the suppression of iNOS and COX-2 at the level of gene transcription. In addition, arvelexin inhibited NF-κB-dependent inflammatory responses by modulating a series of intracellular events of IκB kinase (IKK)-inhibitor κBα (IκBα)-NF-κB signalling. Moreover, arvelexin inhibited IKKβ-elicited NF-κB activation as well as iNOS and COX-2 expression. Serum levels of NO and inflammatory cytokines and mortality in mice challenged injected with LPS were significantly reduced by arvelexin.
Conclusion and implications: Arvelexin down-regulated inflammatory iNOS, COX-2, TNF-α, IL-6 and IL-1β gene expression in macrophages interfering with the activation of IKKβ and p38 mitogen-activated protein kinase, and thus, preventing NF-κB activation.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

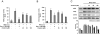
References
-
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. - PubMed
-
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. - PubMed
-
- Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–30863. - PubMed
-
- Corriveau CC, Danner RL. Antiendotoxin therapies for septic shock. Infect Agents Dis. 1993;2:44–52. - PubMed
-
- De Bosscher K, Vanden Berghe W, Vermeulen L, Plaisance S, Boone E, Haegeman G. Glucocorticoids repress NF-kappaB-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proc Natl Acad Sci USA. 2000;97:3919–3924. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

