Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days
- PMID: 21435092
- PMCID: PMC4191844
- DOI: 10.1111/j.1523-5378.2011.00828.x
Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days
Abstract
Background: Ten-day sequential therapy with a proton pump inhibitor (PPI) and amoxicillin followed by a PPI, clarithromycin, and an imidazole typically achieves Helicobacter pylori eradication rates of 90-94% (Grade B success).
Aims: We tested whether prolonging treatment and continuing amoxicillin throughout the 14-day treatment period would produce a ≥ 95% result.
Methods: This was a multicenter pilot study in which H. pylori-infected patients received a 14-day sequential-concomitant hybrid therapy (esomeprazole and amoxicillin for 7 days followed by esomeprazole, amoxicillin clarithromycin, and metronidazole for 7 days). H. pylori status was examined 8 weeks after therapy. Success was defined as achieving ≥ 95% eradication by per-protocol analysis.
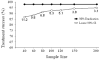
Results: One hundred and seventeen subjects received hybrid therapy. The eradication rate was 99.1% (95% confidence interval (CI), 97.3-100.0%) by per-protocol analysis and 97.4% by intention-to-treat analysis (95% CI, 94.5-100.0%). Adverse events were seen in 14.5%; drug compliance was 94.9%.
Conclusions: Fourteen-day hybrid sequential-concomitant therapy achieved > 95%H. pylori eradication (Grade A result). Further studies are needed 1, in regions with different patterns and frequencies of resistance to confirm these findings, and 2, to examine whether Grade A success is maintained with hybrid therapy shorter than 14 days.
© 2011 Blackwell Publishing Ltd.
Conflict of interest statement
Dr JY Wu discloses no conflicts
Figures
References
-
- Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–25. - PubMed
-
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. - PubMed
-
- Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Iizuka Y, Fukuda K, Fujita Y, Katsurahara M, Ito T, Cesar GE, Imoto I, Takei Y. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter. 2009;14:86–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical