Antiviral propierties of 5,5'-dithiobis-2-nitrobenzoic acid and bacitracin against T-tropic human immunodeficiency virus type 1
- PMID: 21435237
- PMCID: PMC3078101
- DOI: 10.1186/1743-422X-8-137
Antiviral propierties of 5,5'-dithiobis-2-nitrobenzoic acid and bacitracin against T-tropic human immunodeficiency virus type 1
Abstract
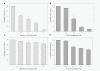
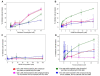
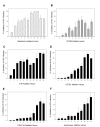
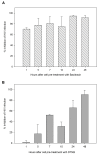
Bacitracin and the membrane-impermeant thiol reagent 5,5'-dithiobis-2-nitrobenzoic acid (DTNB) are agents known to inhibit protein disulfide isomerase (PDI), a cell-surface protein critical in HIV-1 entry therefore they are fusion inhibitors (FI). Here we investigated the possibility that Bacitracin and or DTNB might have other antiviral activities besides FI. By means of residual activity assays, we found that both compounds showed antiviral activity only to viruses T-tropic HIV-1 strain. Cell-based fusion assays showed inhibition on HeLa-CD4-LTR-β-gal (CD4) and HL2/3 cells treated with Bacitracin, and DTNB with the latest compound we observed fusion inhibition on both cells but strikingly in HL2/3 cells (expressing Env) indicating a possible activity on both, the cell membrane and the viral envelope. A time-of-addition experiment showed that both compounds act on HIV entry inhibition but DTNB also acts at late stages of the viral cycle. Lastly, we also found evidence of long-lasting host cell protection in vitro by DTNB, an important pharmacodynamic parameter for a topical microbicide against virus infection, hours after the extracellular drug was removed; this protection was not rendered by Bacitracin. These drugs proved to be leading compounds for further studies against HIV showing antiviral characteristics of interest.
Figures
Similar articles
-
Cell-type specific requirements for thiol/disulfide exchange during HIV-1 entry and infection.Retrovirology. 2012 Dec 3;9:97. doi: 10.1186/1742-4690-9-97. Retrovirology. 2012. PMID: 23206338 Free PMC article.
-
Inhibiting rotavirus infection by membrane-impermeant thiol/disulfide exchange blockers and antibodies against protein disulfide isomerase.Intervirology. 2012;55(6):451-64. doi: 10.1159/000335262. Epub 2012 Mar 7. Intervirology. 2012. PMID: 22398681
-
Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction.Proc Natl Acad Sci U S A. 1994 May 10;91(10):4559-63. doi: 10.1073/pnas.91.10.4559. Proc Natl Acad Sci U S A. 1994. PMID: 8183947 Free PMC article.
-
Small Molecule HIV-1 Attachment Inhibitors: Discovery, Mode of Action and Structural Basis of Inhibition.Viruses. 2021 May 6;13(5):843. doi: 10.3390/v13050843. Viruses. 2021. PMID: 34066522 Free PMC article. Review.
-
Mechanistic Studies of Viral Entry: An Overview of Dendrimer-Based Microbicides As Entry Inhibitors Against Both HIV and HSV-2 Overlapped Infections.Med Res Rev. 2017 Jan;37(1):149-179. doi: 10.1002/med.21405. Epub 2016 Aug 12. Med Res Rev. 2017. PMID: 27518199 Review.
Cited by
-
Candidate antibody-based therapeutics against HIV-1.BioDrugs. 2012 Jun 1;26(3):143-62. doi: 10.2165/11631400-000000000-00000. BioDrugs. 2012. PMID: 22462520 Free PMC article. Review.
-
Cell-type specific requirements for thiol/disulfide exchange during HIV-1 entry and infection.Retrovirology. 2012 Dec 3;9:97. doi: 10.1186/1742-4690-9-97. Retrovirology. 2012. PMID: 23206338 Free PMC article.
-
Synthesis and Biological Activity of Glycosyl Thiazolyl Disulfides Based on Thiacarpine, an Analogue of the Cytotoxic Alkaloid Polycarpine from the Ascidian Polycarpa aurata.Mar Drugs. 2025 Mar 9;23(3):117. doi: 10.3390/md23030117. Mar Drugs. 2025. PMID: 40137303 Free PMC article.
-
Discovery of unsymmetrical aromatic disulfides as novel inhibitors of SARS-CoV main protease: Chemical synthesis, biological evaluation, molecular docking and 3D-QSAR study.Eur J Med Chem. 2017 Sep 8;137:450-461. doi: 10.1016/j.ejmech.2017.05.045. Epub 2017 Jun 9. Eur J Med Chem. 2017. PMID: 28624700 Free PMC article.
-
Functions and mechanisms of protein disulfide isomerase family in cancer emergence.Cell Biosci. 2022 Aug 14;12(1):129. doi: 10.1186/s13578-022-00868-6. Cell Biosci. 2022. PMID: 35965326 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

