Interleukin-1 receptor-mediated inflammation impairs the heat shock response of human mesothelial cells
- PMID: 21435443
- PMCID: PMC3078451
- DOI: 10.1016/j.ajpath.2010.12.034
Interleukin-1 receptor-mediated inflammation impairs the heat shock response of human mesothelial cells
Abstract
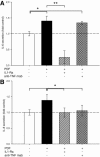
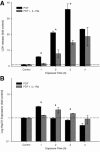
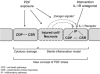
Bioincompatibility of peritoneal dialysis fluids (PDF) limits their use in renal replacement therapy. PDF exposure harms mesothelial cells but induces heat shock proteins (HSP), which are essential for repair and cytoprotection. We searched for cellular pathways that impair the heat shock response in mesothelial cells after PDF-exposure. In a dose-response experiment, increasing PDF-exposure times resulted in rapidly increasing mesothelial cell damage but decreasing HSP expression, confirming impaired heat shock response. Using proteomics and bioinformatics, simultaneously activated apoptosis-related and inflammation-related pathways were identified as candidate mechanisms. Testing the role of sterile inflammation, addition of necrotic cell material to mesothelial cells increased, whereas addition of the interleukin-1 receptor (IL-1R) antagonist anakinra to PDF decreased release of inflammatory cytokines. Addition of anakinra during PDF exposure resulted in cytoprotection and increased chaperone expression. Thus, activation of the IL-1R plays a pivotal role in impairment of the heat shock response of mesothelial cells to PDF. Danger signals from injured cells lead to an elevated level of cytokine release associated with sterile inflammation, which reduces expression of HSP and other cytoprotective chaperones and exacerbates PDF damage. Blocking the IL-1R pathway might be useful in limiting damage during peritoneal dialysis.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Injury-induced inflammation and inadequate HSP expression in mesothelial cells upon repeat exposure to dual-chamber bag peritoneal dialysis fluids.Int J Artif Organs. 2015 Oct;38(10):530-6. doi: 10.5301/ijao.5000438. Epub 2015 Oct 9. Int J Artif Organs. 2015. PMID: 26481292
-
Overexpression of HSP-72 confers cytoprotection in experimental peritoneal dialysis.Kidney Int. 2004 Dec;66(6):2300-7. doi: 10.1111/j.1523-1755.2004.66040.x. Kidney Int. 2004. PMID: 15569319
-
Alanyl-glutamine dipeptide restores the cytoprotective stress proteome of mesothelial cells exposed to peritoneal dialysis fluids.Nephrol Dial Transplant. 2012 Mar;27(3):937-46. doi: 10.1093/ndt/gfr459. Epub 2011 Aug 19. Nephrol Dial Transplant. 2012. PMID: 21856758 Clinical Trial.
-
Biocompatibility and buffers: effect of bicarbonate-buffered peritoneal dialysis fluids on peritoneal cell function.Kidney Int. 1998 Dec;54(6):2184-93. doi: 10.1046/j.1523-1755.1998.00178.x. Kidney Int. 1998. PMID: 9853285 Review.
-
Heat Shock Proteins: Therapeutic Perspectives in Inflammatory Disorders.Recent Pat Inflamm Allergy Drug Discov. 2017;10(2):94-104. doi: 10.2174/1872213X10666161213163301. Recent Pat Inflamm Allergy Drug Discov. 2017. PMID: 27978789 Review.
Cited by
-
Addition of Alanyl-Glutamine to Dialysis Fluid Restores Peritoneal Cellular Stress Responses - A First-In-Man Trial.PLoS One. 2016 Oct 21;11(10):e0165045. doi: 10.1371/journal.pone.0165045. eCollection 2016. PLoS One. 2016. PMID: 27768727 Free PMC article. Clinical Trial.
-
Is there such a thing as biocompatible peritoneal dialysis fluid?Pediatr Nephrol. 2017 Oct;32(10):1835-1843. doi: 10.1007/s00467-016-3461-y. Epub 2016 Oct 8. Pediatr Nephrol. 2017. PMID: 27722783 Free PMC article. Review.
-
Sterile inflammation of peritoneal membrane caused by peritoneal dialysis: focus on the communication between immune cells and peritoneal stroma.Front Immunol. 2024 May 8;15:1387292. doi: 10.3389/fimmu.2024.1387292. eCollection 2024. Front Immunol. 2024. PMID: 38779674 Free PMC article. Review.
-
Functional and Transcriptomic Characterization of Peritoneal Immune-Modulation by Addition of Alanyl-Glutamine to Dialysis Fluid.Sci Rep. 2017 Jul 24;7(1):6229. doi: 10.1038/s41598-017-05872-2. Sci Rep. 2017. PMID: 28740213 Free PMC article. Clinical Trial.
-
Cross-omics comparison of stress responses in mesothelial cells exposed to heat- versus filter-sterilized peritoneal dialysis fluids.Biomed Res Int. 2015;2015:628158. doi: 10.1155/2015/628158. Epub 2015 Oct 1. Biomed Res Int. 2015. PMID: 26495307 Free PMC article.
References
-
- Topley N., Kaur D., Petersen M.M., Jorres A., Williams J.D., Faict D., Holmes C.J. In vitro effects of bicarbonate and bicarbonate-lactate buffered peritoneal dialysis solutions on mesothelial and neutrophil function. J Am Soc Nephrol. 1996;7:218–224. - PubMed
-
- Jorres A., Bender T.O., Finn A., Witowski J., Frohlich S., Gahl G.M., Frei U., Keck H., Passlick-Deetjen J. Biocompatibility and buffers: effect of bicarbonate-buffered peritoneal dialysis fluids on peritoneal cell function. Kidney Int. 1998;54:2184–2193. - PubMed
-
- Witowski J., Topley N., Jorres A., Liberek T., Coles G.A., Williams J.D. Effect of lactate-buffered peritoneal dialysis fluids on human peritoneal mesothelial cell interleukin-6 and prostaglandin synthesis. Kidney Int. 1995;47:282–293. - PubMed
-
- Kratochwill K., Lechner M., Siehs C., Lederhuber H.C., Rehulka P., Endemann M., Kasper D.C., Herkner K.R., Mayer B., Rizzi A., Aufricht C. Stress responses and conditioning effects in mesothelial eells exposed to peritoneal dialysis fluid. J Proteome Res. 2009;8:1731–1747. - PubMed
-
- Lechner M., Kratochwill K., Lichtenauer A., Rehulka P., Mayer B., Aufricht C., Rizzi A. A proteomic view on the role of glucose in peritoneal dialysis. J Proteome Res. 2010;9:2472–2479. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

