Genotypic and phenotypic characterization of side population of gastric cancer cell lines
- PMID: 21435459
- PMCID: PMC3078445
- DOI: 10.1016/j.ajpath.2010.12.043
Genotypic and phenotypic characterization of side population of gastric cancer cell lines
Abstract
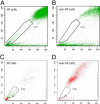
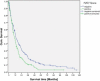
The side population (SP) of tumor cell lines shares characteristics with tumor stem cells. The objective of this study was to phenotypically and genotypically characterize the SP of gastric cancer cell lines. SP cells were obtained from AGS and MKN45 gastric cancer cells using Hoechst 33342 staining and fluorescence-activated cell sorting. The cells were subsequently studied morphologically at cytology and immunocytochemistry, on the transcriptional level via gene array, and in cell culture using recultivation assays. Genes differentially expressed in SP cells were evaluated at immunohistochemistry in tissue samples from 486 patients with gastric cancer. The SP cells were smaller and rounder then non-SP cells. SP cells self-renewed in recultivation experiments and differentiated into SP and non-SP cells. Recultivated SP and non-SP cells exhibited distinct phenotypes in culture insofar as cell shape and colony formation. SP cells demonstrated increased levels of the stem cell markers CD133 and Musashi-1. Transcriptional analyses demonstrated that SP cells express genes that encode for stem cell properties including FZD7, HEY1, SMO, and ADAM17. It was observed that ADAM17 and FZD7 are differentially expressed in human gastric cancer, and FZD7-positive cancers are associated with significantly shorter patient survival. In conclusion, human gastric cancer cell lines enclose a phenotypically and genotypically distinct cell population with tumor stem cell features. Phenotypic characteristics of this distinct cell population are also present in gastric cancer tissue, and correlate with patient survival.
Copyright © 2011 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Paoletti X., Oba K., Burzykowski T., Michiels S., Ohashi Y., Pignon J.P., Rougier P., Sakamoto J., Sargent D., Sasako M., Van Cutsem E., Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. - PubMed
-
- Todaro M., Francipane M.G., Medema J.P., Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–2162. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

