Histologic and biomechanical evaluation of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral incisional hernia repair
- PMID: 21435917
- PMCID: PMC3782991
- DOI: 10.1016/j.jamcollsurg.2011.01.006
Histologic and biomechanical evaluation of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral incisional hernia repair
Abstract
Background: The objective of this study was to evaluate the biomechanical characteristics and histologic remodeling of crosslinked (Peri-Guard, Permacol) and non-crosslinked (AlloDerm, Veritas) biologic meshes over a 12 month period using a porcine model of incisional hernia repair.
Study design: Bilateral incisional hernias were created in 48 Yucatan minipigs and repaired after 21 days using an underlay technique. Samples were harvested at 1, 6, and 12 months and analyzed for biomechanical and histologic properties. The same biomechanical tests were conducted with de novo (time 0) meshes as well as samples of native abdominal wall. Statistical significance (p < 0.05) was determined using 1-way analysis of variance with a Fisher's least significant difference post-test.
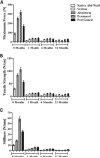
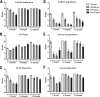
Results: All repair sites demonstrated similar tensile strengths at 1, 6, and 12 months and no significant differences were observed between mesh materials (p > 0.05 in all cases). The strength of the native porcine abdominal wall was not augmented by the presence of the mesh at any of the time points, regardless of de novo tensile strength of the mesh. Histologically, non-crosslinked materials showed earlier cell infiltration (p < 0.01), extracellular matrix deposition (p < 0.02), scaffold degradation (p < 0.05), and neovascularization (p < 0.02) compared with crosslinked materials. However, by 12 months, crosslinked materials showed similar results compared with the non-crosslinked materials for many of the features evaluated.
Conclusions: The tensile strengths of sites repaired with biologic mesh were not impacted by very high de novo tensile strength/stiffness or mesh-specific variables such as crosslinking. Although crosslinking distinguishes biologic meshes in the short-term for histologic features, such as cellular infiltration and neovascularization, many differences diminish during longer periods of time. Characteristics other than crosslinking, such as tissue type and processing conditions, are likely responsible for these differences.
Copyright © 2011 American College of Surgeons. Published by Elsevier Inc. All rights reserved.
Figures
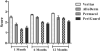

Comment in
-
Evaluation of biologic meshes in a porcine model of ventral incisional hernia repair.J Am Coll Surg. 2011 Nov;213(5):691; author reply 692. doi: 10.1016/j.jamcollsurg.2011.08.002. J Am Coll Surg. 2011. PMID: 22036581 No abstract available.
Similar articles
-
Early biocompatibility of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral hernia repair.Hernia. 2011 Apr;15(2):157-64. doi: 10.1007/s10029-010-0770-0. Epub 2011 Jan 8. Hernia. 2011. PMID: 21222009 Free PMC article.
-
Remodeling characteristics and biomechanical properties of a crosslinked versus a non-crosslinked porcine dermis scaffolds in a porcine model of ventral hernia repair.Hernia. 2015 Apr;19(2):207-18. doi: 10.1007/s10029-013-1070-2. Epub 2013 Mar 13. Hernia. 2015. PMID: 23483265 Free PMC article.
-
Histologic and biomechanical evaluation of a novel macroporous polytetrafluoroethylene knit mesh compared to lightweight and heavyweight polypropylene mesh in a porcine model of ventral incisional hernia repair.Hernia. 2011 Aug;15(4):423-31. doi: 10.1007/s10029-011-0787-z. Epub 2011 Jan 30. Hernia. 2011. PMID: 21279663 Free PMC article.
-
Properties of meshes used in hernia repair: a comprehensive review of synthetic and biologic meshes.J Reconstr Microsurg. 2015 Feb;31(2):83-94. doi: 10.1055/s-0034-1376886. Epub 2014 Sep 5. J Reconstr Microsurg. 2015. PMID: 25192272 Review.
-
A Current Review of Biologic Meshes in Abdominal Wall Reconstruction.Plast Reconstr Surg. 2018 Sep;142(3 Suppl):74S-81S. doi: 10.1097/PRS.0000000000004866. Plast Reconstr Surg. 2018. PMID: 30138272 Review.
Cited by
-
Techniques for Closing the Abdominal Wall in Intestinal and Multivisceral Transplantation: A Systematic Review.Ann Transplant. 2022 Mar 1;27:e934595. doi: 10.12659/AOT.934595. Ann Transplant. 2022. PMID: 35228508 Free PMC article.
-
Tissue response, macrophage phenotype, and intrinsic calcification induced by cardiovascular biomaterials: Can clinical regenerative potential be predicted in a rat subcutaneous implant model?J Biomed Mater Res A. 2022 Feb;110(2):245-256. doi: 10.1002/jbm.a.37280. Epub 2021 Jul 29. J Biomed Mater Res A. 2022. PMID: 34323360 Free PMC article.
-
Laparoscopic ventral/incisional hernia repair: updated Consensus Development Conference based guidelines [corrected].Surg Endosc. 2015 Sep;29(9):2463-84. doi: 10.1007/s00464-015-4293-8. Epub 2015 Jul 3. Surg Endosc. 2015. PMID: 26139480
-
Bioprosthetic tissue matrices in complex abdominal wall reconstruction.Plast Reconstr Surg Glob Open. 2014 Jan 6;1(9):e91. doi: 10.1097/GOX.0000000000000036. eCollection 2013 Dec. Plast Reconstr Surg Glob Open. 2014. PMID: 25289285 Free PMC article.
-
Veritas® bovine pericardium for immediate breast reconstruction: a xenograft alternative to acellular dermal matrix products.Eur J Plast Surg. 2012 Oct;35(10):717-722. doi: 10.1007/s00238-012-0736-9. Epub 2012 Jun 17. Eur J Plast Surg. 2012. PMID: 23002328 Free PMC article.
References
-
- Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg. 1985;72:70–71. - PubMed
-
- Sugerman HJ, Kellum JM, Jr, Reines HD, et al. Greater risk of incisional hernia with morbidly obese than steroid-dependent patients and low recurrence with prefascial polypropylene mesh. Am J Surg. 1996;171:80–84. - PubMed
-
- Hoer J, Lawong G, Klinge U, Schumpelick V. [Factors influencing the development of incisional hernia. A retrospective study of 2,983 laparotomy patients over a period of 10 years]. Chirurg. 2002;73:474–480. - PubMed
-
- Anthony T, Bergen PC, Kim LT, et al. Factors affecting recurrence following incisional herniorrhaphy. World J Surg. 2000;24:95–100. - PubMed
-
- Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343:392–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

