Histone deacetylase inhibitor treatment dramatically reduces cholesterol accumulation in Niemann-Pick type C1 mutant human fibroblasts
- PMID: 21436030
- PMCID: PMC3078401
- DOI: 10.1073/pnas.1014890108
Histone deacetylase inhibitor treatment dramatically reduces cholesterol accumulation in Niemann-Pick type C1 mutant human fibroblasts
Abstract
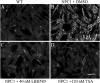
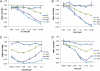
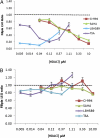
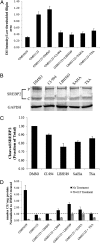
Niemann-Pick type C (NPC) disease is predominantly caused by mutations in the NPC1 protein that affect intracellular cholesterol trafficking and cause accumulation of unesterified cholesterol and other lipids in lysosomal storage organelles. We report the use of a series of small molecule histone deacetylase (HDAC) inhibitors in tissue culture models of NPC human fibroblasts. Some HDAC inhibitors lead to a dramatic correction in the NPC phenotype in cells with either one or two copies of the NPC1(I1061T) mutation, and for several of the inhibitors, correction is associated with increased expression of NPC1 protein. Increased NPC1(I1061T) protein levels may partially account for the correction of the phenotype, because this mutant can promote cholesterol efflux if it is delivered to late endosomes and lysosomes. The HDAC inhibitor treatment is ineffective in an NPC2 mutant human fibroblast line. Analysis of the isoform selectivity of the compounds used implicates HDAC1 and/or HDAC2 as likely targets for the observed correction, although other HDACs may also play a role. LBH589 (panobinostat) is an orally available HDAC inhibitor that crosses the blood-brain barrier and is currently in phase III clinical trials for several types of cancer. It restores cholesterol homeostasis in cultured NPC1 mutant fibroblasts to almost normal levels within 72 h when used at 40 nM. The findings that HDAC inhibitors can correct cholesterol storage defects in human NPC1 mutant cells provide the potential basis for treatment options for NPC disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. - PubMed
-
- Budillon A, Bruzzese F, Di Gennaro E, Caraglia M. Multiple-target drugs: Inhibitors of heat shock protein 90 and of histone deacetylase. Curr Drug Targets. 2005;6:337–351. - PubMed
-
- Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. - PubMed
-
- Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: Overview and perspectives. Mol Cancer Res. 2007;5:981–989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

