Agonist activation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site
- PMID: 21436053
- PMCID: PMC3078348
- DOI: 10.1073/pnas.1017975108
Agonist activation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site
Abstract
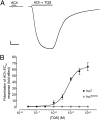
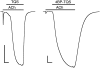
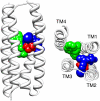
Conventional nicotinic acetylcholine receptor (nAChR) agonists, such as acetylcholine, act at an extracellular "orthosteric" binding site located at the interface between two adjacent subunits. Here, we present evidence of potent activation of α7 nAChRs via an allosteric transmembrane site. Previous studies have identified a series of nAChR-positive allosteric modulators (PAMs) that lack agonist activity but are able to potentiate responses to orthosteric agonists, such as acetylcholine. It has been shown, for example, that TQS acts as a conventional α7 nAChR PAM. In contrast, we have found that a compound with close chemical similarity to TQS (4BP-TQS) is a potent allosteric agonist of α7 nAChRs. Whereas the α7 nAChR antagonist metyllycaconitine acts competitively with conventional nicotinic agonists, metyllycaconitine is a noncompetitive antagonist of 4BP-TQS. Mutation of an amino acid (M253L), located in a transmembrane cavity that has been proposed as being the binding site for PAMs, completely blocks agonist activation by 4BP-TQS. In contrast, this mutation had no significant effect on agonist activation by acetylcholine. Conversely, mutation of an amino acid located within the known orthosteric binding site (W148F) has a profound effect on agonist potency of acetylcholine (resulting in a shift of ∼200-fold in the acetylcholine dose-response curve), but had little effect on the agonist dose-response curve for 4BP-TQS. Computer docking studies with an α7 homology model provides evidence that both TQS and 4BP-TQS bind within an intrasubunit transmembrane cavity. Taken together, these findings provide evidence that agonist activation of nAChRs can occur via an allosteric transmembrane site.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: New twists and turns. Trends Neurosci. 2004;27:329–336. - PubMed
-
- Le Novère N, Changeux J-P. Molecular evolution of the nicotinic acetylcholine receptor: An example of multigene family in excitable cells. J Mol Evol. 1995;40:155–172. - PubMed
-
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. - PubMed
-
- Changeux J-P, Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med. 2008;14:93–102. - PubMed
-
- Weiland S, Bertrand D, Leonard S. Neuronal nicotinic acetylcholine receptors: From the gene to the disease. Behav Brain Res. 2000;113:43–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

