HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis
- PMID: 21436070
- PMCID: PMC3109539
- DOI: 10.1182/blood-2011-01-331306
HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis
Abstract
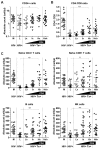
The mechanisms of CD4(+) T-cell count decline, the hallmark of HIV disease progression, and its relationship to elevated levels of immune activation are not fully understood. Massive depletion of CD4(+) T cells occurs during the course of HIV-1 infection, so that maintenance of adequate CD4(+) T-cell levels probably depends primarily on the capacity to renew depleted lymphocytes, that is, the lymphopoiesis. We performed here a comprehensive study of quantitative and qualitative attributes of CD34(+) hematopoietic progenitor cells directly from the blood of a large set of HIV-infected persons compared with uninfected donors, in particular the elderly. Our analyses underline a marked impairment of primary immune resources with the failure to maintain adequate lymphocyte counts. Systemic immune activation emerges as a major correlate of altered lymphopoiesis, which can be partially reversed with prolonged antiretroviral therapy. Importantly, HIV disease progression despite elite control of HIV replication or virologic success on antiretroviral treatment is associated with persistent damage to the lymphopoietic system or exhaustion of lymphopoiesis. These findings highlight the importance of primary hematopoietic resources in HIV pathogenesis and the response to antiretroviral treatments.
Figures


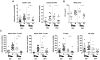



References
-
- Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–870. - PubMed
-
- Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1(4):285–289. - PubMed
-
- Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8(4):319–323. - PubMed
-
- Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17(13):1881–1888. - PubMed
-
- Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104(4):942–947. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

