Focused ultrasound surgery in oncology: overview and principles
- PMID: 21436096
- PMCID: PMC3064817
- DOI: 10.1148/radiol.11100155
Focused ultrasound surgery in oncology: overview and principles
Abstract
Focused ultrasound surgery (FUS) is a noninvasive image-guided therapy and an alternative to surgical interventions. It presents an opportunity to revolutionize cancer therapy and to affect or change drug delivery of therapeutic agents in new focally targeted ways. In this article the background, principles, technical devices, and clinical cancer applications of image-guided FUS are reviewed.
© RSNA, 2011.
Figures

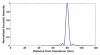











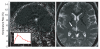
References
-
- Wood RW, Loomis AL. The physical and biological effects of high frequency sound waves of great intensity. Philos Mag Ser 7 1927;4(22):417–436
-
- Seabrook W. Doctor Wood, modern wizard of the laboratory: the story of an American small boy who became the most daring and original experimental physicist of our day—but never grew up. New York, NY: Harcourt, Brace, 1941
-
- Moonen CT. Spatio-temporal control of gene expression and cancer treatment using magnetic resonance imaging-guided focused ultrasound. Clin Cancer Res 2007;13(12):3482–3489 - PubMed
-
- McDannold NJ, Jolesz FA. Magnetic resonance image-guided thermal ablations. Top Magn Reson Imaging 2000;11(3):191–202 - PubMed
-
- Lindner JR, Jr, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 2001;104(17):2107–2112 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

