Characterization of a MAPK scaffolding protein logic gate in gonadotropes
- PMID: 21436256
- PMCID: PMC3100603
- DOI: 10.1210/me.2010-0387
Characterization of a MAPK scaffolding protein logic gate in gonadotropes
Abstract
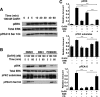
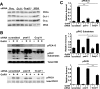
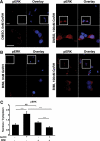
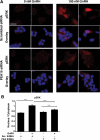
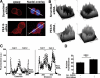
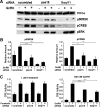
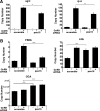
In the pituitary gonadotropes, both protein kinase C (PKC) and MAPK/ERK signaling cascades are activated by GnRH. Phosphoprotein-enriched in astrocytes 15 (PEA-15) is a cytosolic ERK scaffolding protein, which is expressed in LβT2 gonadotrope cells. Pharmacological inhibition of PKC and small interfering RNA-mediated silencing of Gαq/11 revealed that GnRH induces accumulation of phosphorylated PEA-15 in a PKC-dependent manner. To investigate the potential role of PEA-15 in GnRH signaling, we examined the regulation of ERK subcellular localization and the activation of ribosomal S6 kinase, a substrate of ERK. Results obtained by cellular fractionation/Western blot analysis and immunohistochemistry revealed that GnRH-induced accumulation of phosphorylated ERK in the nucleus was attenuated when PEA-15 expression was reduced. Conversely, in the absence of GnRH stimulation, PEA-15 anchors ERK in the cytosol. Our data suggest that GnRH-induced nuclear translocation of ERK requires its release from PEA-15, which occurs upon PEA-15 phosphorylation by PKC. Additional gene-silencing experiments in GnRH-stimulated cells demonstrated that ribosomal S6 kinase activation was dependent on both PEA-15 and PKC. Furthermore, small interfering RNA-mediated knockdown of PEA-15 caused a reduction in GnRH-stimulated expression of early response genes Egr2 and c-Jun, as well as gonadotropin FSHβ-subunit gene expression. PEA-15 knockdown increased LHβ and common α-glycoprotein subunit mRNAs, suggesting a possible role in differential regulation of gonadotropin subunit gene expression. We propose that PEA-15 represents a novel point of convergence of the PKC and MAPK/ERK pathways under GnRH stimulation. PKC, ERK, and PEA-15 form an AND logic gate that shapes the response of the gonadotrope cell to GnRH.
Figures


Similar articles
-
GnRH activates ERK1/2 leading to the induction of c-fos and LHbeta protein expression in LbetaT2 cells.Mol Endocrinol. 2002 Mar;16(3):419-34. doi: 10.1210/mend.16.3.0791. Mol Endocrinol. 2002. PMID: 11875099
-
Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LbetaT2 gonadotropes.Endocrinology. 2010 Oct;151(10):4882-93. doi: 10.1210/en.2009-1483. Epub 2010 Aug 4. Endocrinology. 2010. PMID: 20685880 Free PMC article.
-
The involvement of phosphatidylinositol 3-kinase in gonadotropin-releasing hormone-induced gonadotropin alpha- and FSHbeta-subunit genes expression in clonal gonadotroph LbetaT2 cells.Mol Cell Endocrinol. 2008 Feb 13;283(1-2):1-11. doi: 10.1016/j.mce.2007.12.001. Epub 2007 Dec 8. Mol Cell Endocrinol. 2008. PMID: 18206295
-
Signal transduction pathways and transcription factors involved in the gonadotropin-releasing hormone-stimulated gonadotropin subunit gene expression.Comp Biochem Physiol B Biochem Mol Biol. 2001 Jun;129(2-3):525-32. doi: 10.1016/s1096-4959(01)00356-6. Comp Biochem Physiol B Biochem Mol Biol. 2001. PMID: 11399488 Review.
-
Regulation of gonadotropin subunit genes in tilapia.Comp Biochem Physiol B Biochem Mol Biol. 2001 Jun;129(2-3):489-502. doi: 10.1016/s1096-4959(01)00345-1. Comp Biochem Physiol B Biochem Mol Biol. 2001. PMID: 11399484 Review.
Cited by
-
Molecular regulation of follicle-stimulating hormone synthesis, secretion and action.J Mol Endocrinol. 2018 Apr;60(3):R131-R155. doi: 10.1530/JME-17-0308. Epub 2018 Feb 7. J Mol Endocrinol. 2018. PMID: 29437880 Free PMC article. Review.
-
Phosphoprotein enriched in astrocytes (PEA)-15: a potential therapeutic target in multiple disease states.Pharmacol Ther. 2014 Sep;143(3):265-74. doi: 10.1016/j.pharmthera.2014.03.006. Epub 2014 Mar 20. Pharmacol Ther. 2014. PMID: 24657708 Free PMC article. Review.
-
Actions and Roles of FSH in Germinative Cells.Int J Mol Sci. 2021 Sep 18;22(18):10110. doi: 10.3390/ijms221810110. Int J Mol Sci. 2021. PMID: 34576272 Free PMC article. Review.
-
Protein logic: a statistical mechanical study of signal integration at the single-molecule level.Biophys J. 2012 Sep 5;103(5):1097-107. doi: 10.1016/j.bpj.2012.07.040. Biophys J. 2012. PMID: 23009860 Free PMC article.
-
β-catenin regulates GnRH-induced FSHβ gene expression.Mol Endocrinol. 2013 Feb;27(2):224-37. doi: 10.1210/me.2012-1310. Epub 2012 Dec 4. Mol Endocrinol. 2013. PMID: 23211523 Free PMC article.
References
-
- Alarid ET, Windle JJ, Whyte DB, Mellon PL. 1996. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 - PubMed
-
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. 2001. Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 - PubMed
-
- Thomas P, Mellon PL, Turgeon J, Waring DW. 1996. The LβT2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology 137:2979–2989 - PubMed
-
- Turgeon JL, Kimura Y, Waring DW, Mellon PL. 1996. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol 10:439–450 - PubMed
-
- Windle JJ, Weiner RI, Mellon PL. 1990. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol 4:597–603 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

