Deletion of Otx2 in GnRH neurons results in a mouse model of hypogonadotropic hypogonadism
- PMID: 21436260
- PMCID: PMC3082331
- DOI: 10.1210/me.2010-0271
Deletion of Otx2 in GnRH neurons results in a mouse model of hypogonadotropic hypogonadism
Abstract
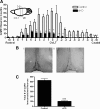
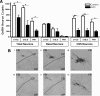
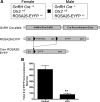
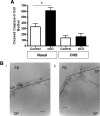
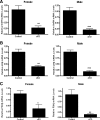
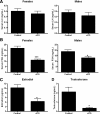
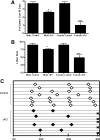
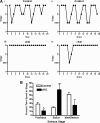
GnRH is the central regulator of reproductive function responding to central nervous system cues to control gonadotropin synthesis and secretion. GnRH neurons originate in the olfactory placode and migrate to the forebrain, in which they are found in a scattered distribution. Congenital idiopathic hypogonadotropic hypogonadism (CIHH) has been associated with mutations or deletions in a number of genes that participate in the development of GnRH neurons and expression of GnRH. Despite the critical role of GnRH in mammalian reproduction, a comprehensive understanding of the developmental factors that are responsible for regulating the establishment of mature GnRH neurons and the expression of GnRH is lacking. orthodenticle homeobox 2 (OTX2), a homeodomain protein required for the formation of the forebrain, has been shown to be expressed in GnRH neurons, up-regulated during GnRH neuronal development, and responsible for increased GnRH promoter activity in GnRH neuronal cell lines. Interestingly, mutations in Otx2 have been associated with human hypogonadotropic hypogonadism, but the mechanism by which Otx2 mutations cause CIHH is unknown. Here we show that deletion of Otx2 in GnRH neurons results in a significant decrease in GnRH neurons in the hypothalamus, a delay in pubertal onset, abnormal estrous cyclicity, and infertility. Taken together, these data provide in vivo evidence that Otx2 is critical for GnRH expression and reproductive competence.
Figures

Similar articles
-
Otx2 induction of the gonadotropin-releasing hormone promoter is modulated by direct interactions with Grg co-repressors.J Biol Chem. 2009 Jun 19;284(25):16966-16978. doi: 10.1074/jbc.M109.002485. Epub 2009 Apr 28. J Biol Chem. 2009. PMID: 19401468 Free PMC article.
-
Deletion of Vax1 from Gonadotropin-Releasing Hormone (GnRH) Neurons Abolishes GnRH Expression and Leads to Hypogonadism and Infertility.J Neurosci. 2016 Mar 23;36(12):3506-18. doi: 10.1523/JNEUROSCI.2723-15.2016. J Neurosci. 2016. PMID: 27013679 Free PMC article.
-
Gene dosage of Otx2 is important for fertility in male mice.Mol Cell Endocrinol. 2013 Sep 5;377(1-2):16-22. doi: 10.1016/j.mce.2013.06.026. Epub 2013 Jun 27. Mol Cell Endocrinol. 2013. PMID: 23811236 Free PMC article.
-
Genetics defects in GNRH1: a paradigm of hypothalamic congenital gonadotropin deficiency.Brain Res. 2010 Dec 10;1364:3-9. doi: 10.1016/j.brainres.2010.09.084. Epub 2010 Sep 29. Brain Res. 2010. PMID: 20887715 Review.
-
The role of semaphorin signaling in the etiology of hypogonadotropic hypogonadism.Minerva Endocrinol. 2016 Jun;41(2):266-78. Epub 2016 Mar 4. Minerva Endocrinol. 2016. PMID: 26940457 Review.
Cited by
-
Autism-linked NLGN3 is a key regulator of gonadotropin-releasing hormone deficiency.Dis Model Mech. 2023 Mar 1;16(3):dmm049996. doi: 10.1242/dmm.049996. Epub 2023 Mar 28. Dis Model Mech. 2023. PMID: 36810932 Free PMC article.
-
Genetic risk variants for metabolic traits in Arab populations.Sci Rep. 2017 Jan 20;7:40988. doi: 10.1038/srep40988. Sci Rep. 2017. PMID: 28106113 Free PMC article.
-
Identification of mRNAs and lncRNAs Involved in the Regulation of Follicle Development in Goat.Front Genet. 2020 Dec 17;11:589076. doi: 10.3389/fgene.2020.589076. eCollection 2020. Front Genet. 2020. PMID: 33391342 Free PMC article.
-
Specification of GnRH-1 neurons by antagonistic FGF and retinoic acid signaling.Dev Biol. 2012 Feb 15;362(2):254-62. doi: 10.1016/j.ydbio.2011.12.016. Epub 2011 Dec 19. Dev Biol. 2012. PMID: 22200593 Free PMC article.
-
Kisspeptin Induces Dynamic Chromatin Modifications to Control GnRH Gene Expression.Mol Neurobiol. 2016 Jul;53(5):3315-3325. doi: 10.1007/s12035-015-9269-0. Epub 2015 Jun 17. Mol Neurobiol. 2016. PMID: 26081144
References
-
- Schwanzel-Fukuda M, Bick D, Pfaff DW. 1989. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res 6:311–326 - PubMed
-
- Cattanach BM, Iddon CA, Charlton HM, Chippa SA, Fink G. 1977. Gonadotropin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269:338–340 - PubMed
-
- Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, Nikolics K, Seeburg PH. 1986. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science 234:1366–1371 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

