Mouse and human neutrophils induce anaphylaxis
- PMID: 21436586
- PMCID: PMC3069785
- DOI: 10.1172/JCI45232
Mouse and human neutrophils induce anaphylaxis
Abstract
Anaphylaxis is a life-threatening hyperacute immediate hypersensitivity reaction. Classically, it depends on IgE, FcεRI, mast cells, and histamine. However, anaphylaxis can also be induced by IgG antibodies, and an IgG1-induced passive type of systemic anaphylaxis has been reported to depend on basophils. In addition, it was found that neither mast cells nor basophils were required in mouse models of active systemic anaphylaxis. Therefore, we investigated what antibodies, receptors, and cells are involved in active systemic anaphylaxis in mice. We found that IgG antibodies, FcγRIIIA and FcγRIV, platelet-activating factor, neutrophils, and, to a lesser extent, basophils were involved. Neutrophil activation could be monitored in vivo during anaphylaxis. Neutrophil depletion inhibited active, and also passive, systemic anaphylaxis. Importantly, mouse and human neutrophils each restored anaphylaxis in anaphylaxis-resistant mice, demonstrating that neutrophils are sufficient to induce anaphylaxis in mice and suggesting that neutrophils can contribute to anaphylaxis in humans. Our results therefore reveal an unexpected role for IgG, IgG receptors, and neutrophils in anaphylaxis in mice. These molecules and cells could be potential new targets for the development of anaphylaxis therapeutics if the same mechanism is responsible for anaphylaxis in humans.
Figures



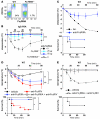

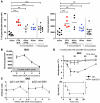
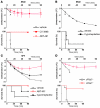

Comment in
-
Neutrophils give us a shock.J Clin Invest. 2011 Apr;121(4):1260-3. doi: 10.1172/JCI57296. Epub 2011 Mar 23. J Clin Invest. 2011. PMID: 21436575 Free PMC article.
-
Allergy: shocking behaviour.Nat Rev Immunol. 2011 May;11(5):302-3. doi: 10.1038/nri2980. Nat Rev Immunol. 2011. PMID: 21508976 No abstract available.
References
-
- Portier P, Richet C. De l’action anaphylactique de certain venins. CR Soc Biol (Paris). 1902;54:170.
-
- Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99(5):901–914. doi: 10.1172/JCI119255. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

