Blood-brain barrier breakdown following traumatic brain injury: a possible role in posttraumatic epilepsy
- PMID: 21436875
- PMCID: PMC3056210
- DOI: 10.1155/2011/765923
Blood-brain barrier breakdown following traumatic brain injury: a possible role in posttraumatic epilepsy
Abstract
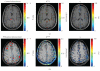
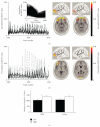
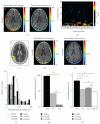
Recent animal experiments indicate a critical role for opening of the blood-brain barrier (BBB) in the pathogenesis of post-traumatic epilepsy (PTE). This study aimed to investigate the frequency, extent, and functional correlates of BBB disruption in epileptic patients following mild traumatic brain injury (TBI). Thirty-seven TBI patients were included in this study, 19 of whom suffered from PTE. All underwent electroencephalographic (EEG) recordings and brain magnetic resonance imaging (bMRI). bMRIs were evaluated for BBB disruption using novel quantitative techniques. Cortical dysfunction was localized using standardized low-resolution brain electromagnetic tomography (sLORETA). TBI patients displayed significant EEG slowing compared to controls with no significant differences between PTE and nonepileptic patients. BBB disruption was found in 82.4% of PTE compared to 25% of non-epileptic patients (P = .001) and could be observed even years following the trauma. The volume of cerebral cortex with BBB disruption was significantly larger in PTE patients (P = .001). Slow wave EEG activity was localized to the same region of BBB disruption in 70% of patients and correlated to the volume of BBB disrupted cortex. We finally present a patient suffering from early cortical dysfunction and BBB breakdown with a gradual and parallel resolution of both pathologies. Our findings demonstrate that BBB pathology is frequently found following mild TBI. Lasting BBB breakdown is found with increased frequency and extent in PTE patients. Based on recent animal studies and the colocalization found between the region of disrupted BBB and abnormal EEG activity, we suggest a role for a vascular lesion in the pathogenesis of PTE.
Figures
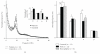
References
-
- Annegers JF, Grabow JD, Groover RV, Laws ER, Jr., Elveback LR, Kurland LT. Seizures after head trauma: a population study. Neurology. 1980;30(7):683–689. - PubMed
-
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. The New England Journal of Medicine. 1998;338(1):20–24. - PubMed
-
- Desai BT, Whitman S, Coonley Hoganson R, Coleman TE, Gabriel G, Dell J. Seizures and civilian head injuries. Epilepsia. 1983;24(3):289–296. - PubMed
-
- Caveness WF, Meirowsky AM, Rish BL, et al. The nature of posttraumatic epilepsy. Journal of Neurosurgery. 1979;50(5):545–553. - PubMed
-
- Asikainen I, Kaste M, Sarna S. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 1999;40(5):584–589. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

