Critical appraisal of the safety and efficacy of insulin detemir in glycemic control and cardiovascular risk management in diabetics
- PMID: 21437089
- PMCID: PMC3047990
- DOI: 10.2147/dmsott.s7315
Critical appraisal of the safety and efficacy of insulin detemir in glycemic control and cardiovascular risk management in diabetics
Abstract
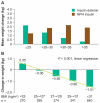
Insulin detemir is an analog of human insulin designed to provide a long duration of basal insulin action. This is achieved by protracted absorption from the injection depot, which results in part from increased self-association of insulin detemir molecules and in part from reversible albumin binding. Subsequent albumin binding in the circulation is thought to buffer changes in the effects at target tissues that could otherwise arise from variability in absorption rate. In consequence, insulin detemir has shown a less variable pharmacodynamic profile than alternative basal insulins; this manifests as more consistent temporal glucose reduction profiles in repeat-clamp studies. In clinical trials, insulin detemir has been characterized by consistent risk reductions in hypoglycemia, as well as reduced weight gain in comparison with other basal insulins. Given some recent associations that have been made in prospective and epidemiologic studies between glucose variability and/or hypoglycemia and increased cardiovascular risk, and the long-known association between excess weight and cardiovascular risk, it is possible that the clinical profile of insulin detemir may carry prognostic value with regard to cardiovascular safety, although this is yet to be substantiated. There have also been some concerns raised recently over the use of insulin analogs and cancer risk, but available clinical data and the receptor interaction profile of insulin detemir suggest no excess in risk in comparison with human insulin therapy. Optimal approaches for the clinical use of insulin detemir have been emerging through an increasing clinical study base, and the analog is becoming established as a potentially valuable therapy option.
Keywords: glucose variability; hypoglycemia; insulin detemir; type 2 diabetes; weight gain.
Figures

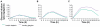
References
-
- AACE. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocrine Pract. 2007;13 (Suppl 1):S1–S68. - PubMed
-
- Rolla AR. Addressing the need to tailor treatment to the spectrum of type 2 diabetes: New perspectives. Diabetes Technol Ther. 2009;11:267–274. - PubMed
-
- ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. - PubMed
LinkOut - more resources
Full Text Sources

