Amniotic-Fluid Stem Cells: Growth Dynamics and Differentiation Potential after a CD-117-Based Selection Procedure
- PMID: 21437196
- PMCID: PMC3062157
- DOI: 10.4061/2011/715341
Amniotic-Fluid Stem Cells: Growth Dynamics and Differentiation Potential after a CD-117-Based Selection Procedure
Abstract
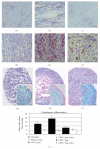
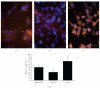
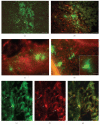
Amniotic fluid (AF) has become an interesting source of fetal stem cells. However, AF contains heterogeneous and multiple, partially differentiated cell types. After isolation from the amniotic fluid, cells were characterized regarding their morphology and growth dynamics. They were sorted by magnetic associated cell sorting using the surface marker CD 117. In order to show stem cell characteristics such as pluripotency and to evaluate a possible therapeutic application of these cells, AF fluid-derived stem cells were differentiated along the adipogenic, osteogenic, and chondrogenic as well as the neuronal lineage under hypoxic conditions. Our findings reveal that magnetic associated cell sorting (MACS) does not markedly influence growth characteristics as demonstrated by the generation doubling time. There was, however, an effect regarding an altered adipogenic, osteogenic, and chondrogenic differentiation capacity in the selected cell fraction. In contrast, in the unselected cell population neuronal differentiation is enhanced.
Figures




References
-
- Hemberger M, Yang W, Natale D, et al. Stem cells from fetal membranes—a workshop report. Placenta. 2008;29:S17–S19. - PubMed
-
- de Coppi P, Bartsch G, Jr., Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology. 2007;25(1):100–106. - PubMed
-
- Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschlager M. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Human Reproduction. 2003;18(7):1489–1493. - PubMed
-
- Gosden CM. Amniotic fluid cell types and culture. British Medical Bulletin. 1983;39(4):348–354. - PubMed
-
- Hoehn H, Salk D. Morphological and biochemical heterogeneity of amniotic fluid cells in culture. Methods in Cell Biology. 1982;26:12–34. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

