Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer
- PMID: 21437893
- PMCID: PMC3174272
- DOI: 10.1002/cncr.26080
Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer
Abstract
Background: The authors sought to improve the toxicity of conventional concurrent chemoradiation therapy for stage III nonsmall cell lung cancer (NSCLC) by using proton-beam therapy to escalate the radiation dose to the tumor. They report early results of a phase 2 study of high-dose proton therapy and concurrent chemotherapy in terms of toxicity, failure patterns, and survival.
Methods: Forty-four patients with stage III NSCLC were treated with 74 grays (radiobiologic equivalent) proton therapy with weekly carboplatin (area under the curve, 2 U) and paclitaxel (50 mg/m(2)). Disease was staged with positron emission tomography/computed tomography (CT), and treatments were simulated with 4-dimensional (4D) CT to account for tumor motion. Protons were delivered as passively scattered beams, and treatment simulation was repeated during the treatment process to determine the need for adaptive replanning.
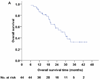
Results: Median follow-up time was 19.7 months (range, 6.1-44.4 months), and median overall survival time was 29.4 months. No patient experienced grade 4 or 5 proton-related adverse events. The most common nonhematologic grade 3 toxicities were dermatitis (n = 5), esophagitis (n = 5), and pneumonitis (n = 1). Nine (20.5%) patients experienced local disease recurrence, but only 4 (9.1%) had isolated local failure. Four (9.1%) patients had regional lymph node recurrence, but only 1 (2.3%) had isolated regional recurrence. Nineteen (43.2%) patients developed distant metastasis. The overall survival and progression-free survival rates were 86% and 63% at 1 year.
Conclusions: Concurrent high-dose proton therapy and chemotherapy are well tolerated, and the median survival time of 29.4 months is encouraging for unresectable stage III NSCLC.
Copyright © 2011 American Cancer Society.
Figures


References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Curran W, Scott C, Langer C, et al. Long term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresectable NSCLC: RTOG 9410 (abstract) Proc Am Soc Clin Oncol. 2003:621a.
-
- Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. - PubMed
-
- Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 study. J Clin Oncol. 2005;23:5910–5917. - PubMed
-
- Socinski M, Rosenman J, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B non-small-cell lung carcinoma. Cancer. 2001;92:1213–1223. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

