Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors
- PMID: 21438154
- PMCID: PMC3377073
- DOI: 10.1002/emmm.201100134
Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors
Abstract
Cold hypersensitivity is the hallmark of oxaliplatin-induced neuropathy, which develops in nearly all patients under this chemotherapy. To date, pain management strategies have failed to alleviate these symptoms, hence development of adapted analgesics is needed. Here, we report that oxaliplatin exaggerates cold perception in mice as well as in patients. These symptoms are mediated by primary afferent sensory neurons expressing the thermoreceptor TRPM8. Mechanistically, oxaliplatin promotes over-excitability by drastically lowering the expression of distinct potassium channels (TREK1, TRAAK) and by increasing the expression of pro-excitatory channels such as the hyperpolarization-activated channels (HCNs). These findings are corroborated by the analysis of TREK1-TRAAK null mice and use of the specific HCN inhibitor ivabradine, which abolishes the oxaliplatin-induced cold hypersensibility. These results suggest that oxaliplatin exacerbates cold perception by modulating the transcription of distinct ionic conductances that together shape sensory neuron responses to cold. The translational and clinical implication of these findings would be that ivabradine may represent a tailored treatment for oxaliplatin-induced neuropathy.
Copyright © 2011 EMBO Molecular Medicine.
Figures
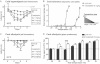
Withdrawal thresholds to tail immersion at 10°C measured daily for 6 days before treatment (day 0) and after single i.p. injection with vehicle (filled circles, n = 10) or 1, 3 or 6 mg/kg of oxaliplatin (open triangle, open square and open circle, respectively; n = 10 per group). The dotted line at 15 s represents the test cut off value.
Dynamic cold plate test performed 90 h after vehicle/oxaliplatin injection. The number of nocifensive reactions (jumps) was measured from 30 to 1°C (vehicle: filled circles; oxaliplatin 6 mg/kg open circles; n = 8 per group).
Withdrawal thresholds to tail immersion at 21°C measured daily for 6 days in mice before (day 0) and after single i.p. injection with vehicle (filled circles, n = 10) or 1, 3 or 6 mg/kg of oxaliplatin (open triangle, open square and open circle, respectively; n = 10 per group).
Thermic place preference at 90 h post vehicle/oxaliplatin injection. Mice were allowed to choose between adjacent surfaces set to 25°C versus a range of temperatures as shown. The percentage of time spent at 25°C over a 3 min period is shown. Filled and open bars represent the vehicle and the oxaliplatin (6 mg/kg) groups, respectively (n = 10 mice per group).

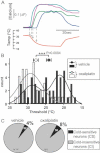
Time course of intracellular calcium elevation in the four cold-sensitive neurons showing the variability in temperature thresholds (represented by grey arrows).
Histogram of frequency distribution of temperature thresholds for cold-sensitive neurons from vehicle- or oxaliplatin-treated mice (dark and open bars, respectively). Distributions of thresholds from vehicle- or oxaliplatin-treated cold-sensitive neurons are fitted, respectively, with a double or a single Gaussian equation. Mean thresholds (±SEM) of vehicle (filled circle: 24.2 ± 0.7°C) and oxaliplatin (open circle: 27.7 ± 0.7°C) are displayed behind the histogram (p = 0.0004).
Effect of oxaliplatin or vehicle on the percentage of cold-sensitive neurons in the total number of DRG cells analysed.
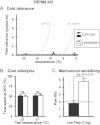
Dynamic cold plate test performed before (filled circles) and 90 h after oxaliplatin injection (open circles, n = 10). Nocifensive reactions were measured from 22 to 1°C. Grey dotted lines represent the reactions of vehicle- and oxaliplatin-treated wild type mice.
Thermal place preference before (filled bars) and 90 h after oxaliplatin injection (open bars, n = 10). Mice were allowed to choose between adjacent surfaces adjusted to 25°C versus 23°C or 21°C.
Effect of oxaliplatin on mechanical perception on the same TRPM8 KO mice as in (A) and (B) (n = 10 per group). Numbers of paw lifts out of 5 mechanical stimulations using a von Frey filament of 1.4 g bending force.
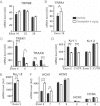
A,B. Expression of the cold-activated thermo receptors, TRPM8 (A) and TRPA1 (B).
C,D. Expression profile of the potassium channels TREEK1 and TRAAK (C), and KV1.1 and KV1.2 (D).
E. Expression of the sodium channel NaV1.8.
F. Expression profile of the HCN1-4 hyperpolarization activated cationic channels.
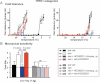
Lack of effect of TRPA1 channel blockade with acute HC030031 treatment on oxaliplatin cold hyperalgesia (left panel). The same treatment reduces normal cold tolerance in control mice (right panel).
Reversal of oxaliplatin-mediated mechanical hyperalgesia by HC030031 in similar experimental conditions as in (A) (n = 20 or 10 per group). Numbers of paw lifts out of five mechanical stimulations using a von Frey filament of 1.4 g bending force.
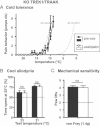
Dynamic cold plate test performed before (filled circles, n = 10) and 90 h after oxaliplatin injection (open circles, n = 10). Nocifensive reactions were measured from 22 to 1°C.
Thermal place preference before (filled bars) and 90 h after oxaliplatin injection (open bars, n = 10). Mice were allowed to choose between adjacent surfaces adjusted to 25°C versus 23°C or 21°C.
Effect of oxaliplatin on mechanical perception on the same TREK1-TRAAK KO mice as in (A) and (B) (n = 10 per group). Numbers of paw lifts out of five mechanical stimulations using a von Frey filament of 1.4 g bending force.
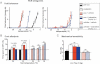
Effect of HCN channel blockade with acute ivabradine treatment on oxaliplatin-induced cold hyperalgesia measured on the dynamic cold plate (left panel). The same treatment minimally affects normal cold tolerance (right panel).
Acute ivabradine treatment reverses cool allodynia measured in the thermal place preference test for two temperature choices (25 versus 23 or 21°C) whilst it does not affect place preference in control animal (25 versus 23, 21 or 19°C) (n = 8 per group).
Lack of effect of ivabradine on oxaliplatin-mediated mechanical hyperalgesia or on acute mechanical perception in similar experimental conditions as in (A) and (B) (n = 8 per group). Numbers of paw lifts out of five mechanical stimulations using a von Frey filament of 1.4 g bending force.
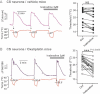
Time course of intracellular calcium elevation in a cold-sensitive neuron from a vehicle-treated animal showing that cooling elicits elevations of intracellular calcium with reproducible thresholds that are not affected by ivabradine treatment. The histogram on the right shows the thresholds before and during ivabradine perfusion for all the cells tested with no statistical differences (n = 18).
Same representation as in (A) for cold-sensitive DRG neurons from oxaliplatin-treated mice. The time course shows that ivabradine alters the cold threshold. The histogram displaying all cold-sensitive neurons tested reveals a significant effect of ivabradine (p = 0.0008, n = 18).

Monomodal cold-specific fibres use TRPM8 as the main detector of innocuous cool and noxious cold stimuli. Oxaliplatin modifies their excitability by decreasing inhibitory potassium channels and increasing excitatory channels with a prominent effect on HCN1.
Polymodal cold and mechanosensitive fibres affected by oxaliplatin also use TRPM8 as cold detector in addition to yet to be identified excitatory mechanosensors. Distinct from cold specific fibres, HCN channels are not present in these neurons reflecting the lack of ivabradine effect in mechanical pain and the incomplete reversal of cold tolerance.
Mechanosensitive fibres with up-regulated TRPA1 and down-regulated K2P in their mechanosensory machinery convey oxaliplatin-mediated mechanical hypersensitivity.
References
-
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. - PubMed
-
- Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan P, Bridgewater J, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. - PubMed
-
- Arvidsson B, Kristensson K, Olsson Y. Vascular permeability to fluorescent protein tracer in trigeminal nerve and gasserian ganglion. Acta Neuropathol. 1973;26:199–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

