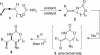Allene functionalization via bicyclic methylene aziridines
- PMID: 21438516
- PMCID: PMC3090038
- DOI: 10.1021/ol2002418
Allene functionalization via bicyclic methylene aziridines
Abstract
The oxidative functionalization of olefins is a common method for the formation of vicinal carbon-heteroatom bonds. However, oxidative methods to transform allenes into synthetic motifs containing three contiguous carbon-heteroatom bonds are much less developed. This paper describes the use of bicyclic methylene aziridines (MAs), prepared via intramolecular allene aziridination, as scaffolds for functionalization of all three allene carbons.
© 2011 American Chemical Society
Figures
References
-
-
For selected reviews on the aziridination of olefins, see: Pellissier H. Tetrahedron. 2010;66:1509.. Sweeney JB. Chem. Soc. Rev. 2002;31:247.. Yudin AK, editor. Aziridines and Epoxides in Organic Synthesis. Wiley-VCH; Weinheim: 2004. . Muller P, Fruit C. Chem. Rev. 2003;103:2905.
-
-
-
For selected references on C-O bond-forming reactions with allenes, see: Katukojvala S, Barlett KN, Lotesta SD, Williams LJ. J. Am. Chem. Soc. 2004;126:15348.. Ghosh P, Lotesta SD, Williams LJ. J. Am. Chem. Soc. 2007;129:2438.. Lotesta SD, Kiren S, Sauers RR, Williams LJ. Angew. Chem. Int. Ed. 2007;46:7108.. Ghosh P, Cusick JR, Inghrim J, Williams LJ. Org. Lett. 2009;11:4672.
-
-
- Robertson J, Feast GC, White LV, Steadman VA, Claridge TDW. Org. Biomol. Chem. 2010;8:3060. - PubMed
- Liu R. Ph.D. Dissertation. Brigham Young University; 2007.
- Atkinson RS, Malpass JR. Tetrahedron Lett. 1975:4305.
- Bingham EM, Gilbert JC. J. Org. Chem. 1975;40:224.
-
- Shipman M. Synlett. 2006:3205.
-
- Hayes CJ, Beavis PW, Humphries LA. Chem. Commun. 2006:4501. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources