Reward, addiction, withdrawal to nicotine
- PMID: 21438686
- PMCID: PMC3137256
- DOI: 10.1146/annurev-neuro-061010-113734
Reward, addiction, withdrawal to nicotine
Abstract
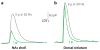
Nicotine is the principal addictive component that drives continued tobacco use despite users' knowledge of the harmful consequences. The initiation of addiction involves the mesocorticolimbic dopamine system, which contributes to the processing of rewarding sensory stimuli during the overall shaping of successful behaviors. Acting mainly through nicotinic receptors containing the α4 and β2 subunits, often in combination with the α6 subunit, nicotine increases the firing rate and the phasic bursts by midbrain dopamine neurons. Neuroadaptations arise during chronic exposure to nicotine, producing an altered brain condition that requires the continued presence of nicotine to be maintained. When nicotine is removed, a withdrawal syndrome develops. The expression of somatic withdrawal symptoms depends mainly on the α5, α2, and β4 (and likely α3) nicotinic subunits involving the epithalamic habenular complex and its targets. Thus, nicotine taps into diverse neural systems and an array of nicotinic acetylcholine receptor (nAChR) subtypes to influence reward, addiction, and withdrawal.
Figures

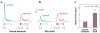
References
-
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates gamma-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–411. - PubMed
-
- Am. Psychiatr. Assoc. (APA) American Psychiatric Association, Diagnostic and Statistical Manual—IVTR. Washington, DC: APA; 2000.
-
- Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Brain Res Rev. 1994;19:1–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

