Macrophage cholesterol homeostasis and metabolic diseases: critical role of cholesteryl ester mobilization
- PMID: 21438812
- PMCID: PMC3098044
- DOI: 10.1586/erc.11.16
Macrophage cholesterol homeostasis and metabolic diseases: critical role of cholesteryl ester mobilization
Abstract
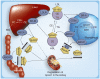
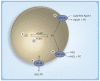
Atherogenic dyslipidemia, including low HDL levels, is the major contributor of residual risk of cardiovascular disease that remains even after aggressive statin therapy to reduce LDL-cholesterol. Currently, distinction is not made between HDL-cholesterol and HDL, which is a lipoprotein consisting of several proteins and a core containing cholesteryl esters (CEs). The importance of assessing HDL functionality, specifically its role in facilitating cholesterol efflux from foam cells, is relevant to atherogenesis. Since HDLs can only remove unesterified cholesterol from macrophages while cholesterol is stored as CEs within foam cells, intracellular CE hydrolysis by CE hydrolase is vital. Reduction in macrophage lipid burden not only attenuates atherosclerosis but also reduces inflammation and linked pathologies such as Type 2 diabetes and chronic kidney disease. Targeting reduction in macrophage CE levels and focusing on enhancing cholesterol flux from peripheral tissues to liver for final elimination is proposed.
Figures


Similar articles
-
Macrophage cholesteryl ester mobilization and atherosclerosis.Vascul Pharmacol. 2010 Jan-Feb;52(1-2):1-10. doi: 10.1016/j.vph.2009.10.002. Epub 2009 Oct 28. Vascul Pharmacol. 2010. PMID: 19878739 Free PMC article. Review.
-
Cooperation between hepatic cholesteryl ester hydrolase and scavenger receptor BI for hydrolysis of HDL-CE.J Lipid Res. 2013 Nov;54(11):3078-84. doi: 10.1194/jlr.M040998. Epub 2013 Aug 29. J Lipid Res. 2013. PMID: 23990661 Free PMC article.
-
Impaired mobilisation of cholesterol from stored cholesteryl esters in human (THP-1) macrophages.Atherosclerosis. 1996 Feb;120(1-2):135-45. doi: 10.1016/0021-9150(95)05695-5. Atherosclerosis. 1996. PMID: 8645354
-
Oxidized low density lipoprotein leads to macrophage accumulation of unesterified cholesterol as a result of lysosomal trapping of the lipoprotein hydrolyzed cholesteryl ester.J Lipid Res. 1994 May;35(5):803-19. J Lipid Res. 1994. PMID: 8071603
-
Roles of peripheral lipoproteins and cholesteryl ester transfer protein in the vascular contributions to cognitive impairment and dementia.Mol Neurodegener. 2023 Nov 16;18(1):86. doi: 10.1186/s13024-023-00671-y. Mol Neurodegener. 2023. PMID: 37974180 Free PMC article. Review.
Cited by
-
Long-term prognostic factors for cardiovascular events in patients with chest pain without diabetes mellitus nor significant coronary stenosis.Heart Vessels. 2024 May;39(5):382-391. doi: 10.1007/s00380-023-02348-4. Epub 2024 Feb 7. Heart Vessels. 2024. PMID: 38324195
-
Update in therapeutic approaches to plaque stabilization.Curr Atheroscler Rep. 2014 Mar;16(3):392. doi: 10.1007/s11883-013-0392-y. Curr Atheroscler Rep. 2014. PMID: 24425061 Review.
-
Nur77 decreases atherosclerosis progression in apoE(-/-) mice fed a high-fat/high-cholesterol diet.PLoS One. 2014 Jan 31;9(1):e87313. doi: 10.1371/journal.pone.0087313. eCollection 2014. PLoS One. 2014. PMID: 24498071 Free PMC article.
-
Cardiovascular disease models: A game changing paradigm in drug discovery and screening.Biomaterials. 2019 Apr;198:3-26. doi: 10.1016/j.biomaterials.2018.09.036. Epub 2018 Oct 1. Biomaterials. 2019. PMID: 30343824 Free PMC article. Review.
-
Physiopathology of necrobiotic xanthogranuloma with monoclonal gammopathy.J Intern Med. 2014 Sep;276(3):269-84. doi: 10.1111/joim.12195. Epub 2014 Feb 10. J Intern Med. 2014. PMID: 24428816 Free PMC article.
References
-
- D’Agostino RB, Russell MW, Huse DM, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am. Heart J. 2000;139(2 Pt 1):272–281. - PubMed
-
- Greenland P, Knoll MD, Stamler J, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290(7):891–897. - PubMed
-
- Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. - PubMed
-
- Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
