Nuclear envelope structural defects cause chromosomal numerical instability and aneuploidy in ovarian cancer
- PMID: 21439080
- PMCID: PMC3072346
- DOI: 10.1186/1741-7015-9-28
Nuclear envelope structural defects cause chromosomal numerical instability and aneuploidy in ovarian cancer
Abstract
Background: Despite our substantial understanding of molecular mechanisms and gene mutations involved in cancer, the technical approaches for diagnosis and prognosis of cancer are limited. In routine clinical diagnosis of cancer, the procedure is very basic: nuclear morphology is used as a common assessment of the degree of malignancy, and hence acts as a prognostic and predictive indicator of the disease. Furthermore, though the atypical nuclear morphology of cancer cells is believed to be a consequence of oncogenic signaling, the molecular basis remains unclear. Another common characteristic of human cancer is aneuploidy, but the causes and its role in carcinogenesis are not well established.
Methods: We investigated the expression of the nuclear envelope proteins lamin A/C in ovarian cancer by immunohistochemistry and studied the consequence of lamin A/C suppression using siRNA in primary human ovarian surface epithelial cells in culture. We used immunofluorescence microscopy to analyze nuclear morphology, flow cytometry to analyze cellular DNA content, and fluorescence in situ hybridization to examine cell ploidy of the lamin A/C-suppressed cells.
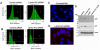
Results: We found that nuclear lamina proteins lamin A/C are often absent (47%) in ovarian cancer cells and tissues. Even in lamin A/C-positive ovarian cancer, the expression is heterogeneous within the population of tumor cells. In most cancer cell lines, a significant fraction of the lamin A/C-negative population was observed to intermix with the lamin A/C-positive cells. Down regulation of lamin A/C in non-cancerous primary ovarian surface epithelial cells led to morphological deformation and development of aneuploidy. The aneuploid cells became growth retarded due to a p53-dependent induction of the cell cycle inhibitor p21.
Conclusions: We conclude that the loss of nuclear envelope structural proteins, such as lamin A/C, may underlie two of the hallmarks of cancer--aberrations in nuclear morphology and aneuploidy.
Figures
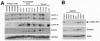




References
-
- Dehari R, Kurman RJ, Logani S, Shih IeM. The development of high-grade serous carcinoma from atypical proliferative (borderline) serous tumors and low-grade micropapillary serous carcinoma: a morphologic and molecular genetic analysis. Am J Surg Pathol. 2007;31:1007–1012. doi: 10.1097/PAS.0b013e31802cbbe9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

