Serum amyloid A 2.2 refolds into a octameric oligomer that slowly converts to a more stable hexamer
- PMID: 21439938
- PMCID: PMC3246010
- DOI: 10.1016/j.bbrc.2011.03.090
Serum amyloid A 2.2 refolds into a octameric oligomer that slowly converts to a more stable hexamer
Abstract
Serum amyloid A (SAA) is an inflammatory protein predominantly bound to high-density lipoprotein in plasma and presumed to play various biological and pathological roles. We previously found that the murine isoform SAA2.2 exists in aqueous solution as a marginally stable hexamer at 4-20°C, but becomes an intrinsically disordered protein at 37°C. Here we show that when urea-denatured SAA2.2 is dialyzed into buffer (pH 8.0, 4°C), it refolds mostly into an octameric species. The octamer transitions to the hexameric structure upon incubation from days to weeks at 4°C, depending on the SAA2.2 concentration. Thermal denaturation of the octamer and hexamer monitored by circular dichroism showed that the octamer is ∼10°C less stable, with a denaturation mid point of ∼22°C. Thus, SAA2.2 becomes kinetically trapped by refolding into a less stable, but more kinetically accessible octameric species. The ability of SAA2.2 to form different oligomeric species in vitro along with its marginal stability, suggest that the structure of SAA might be modulated in vivo to form different biologically relevant species.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



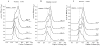
References
-
- Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. - PubMed
-
- Aldo-Benson MA, Benson MD. SAA suppression of immune response in vitro: evidence for an effect on T cell-macrophage interaction. J Immunol. 1982;128:2390–2392. - PubMed
-
- Steinmetz A, Hocke G, Saile R, Puchois P, Fruchart JC. Influence of serum amyloid A on cholesterol esterification in human plasma. Biochim Biophys Acta. 1989;1006:173–178. - PubMed
-
- Kisilevsky R, Subrahmanyan L. Serum amyloid A changes high density lipoprotein’s cellular affinity. A clue to serum amyloid A’s principal function. Lab Invest. 1992;66:778–785. - PubMed
-
- Liang JS, Schreiber BM, Salmona M, Phillip G, Gonnerman WA, de Beer FC, Sipe JD. Amino terminal region of acute phase, but not constitutive, serum amyloid A (apoSAA) specifically binds and transports cholesterol into aortic smooth muscle and HepG2 cells. J Lipid Res. 1996;37:2109–2116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

