TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis
- PMID: 21439959
- PMCID: PMC3129497
- DOI: 10.1053/j.gastro.2011.03.041
TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis
Abstract
Background & aims: Acute pancreatitis is characterized by early activation of intracellular proteases followed by acinar cell death and inflammation. Activation of damage-associated molecular pattern (DAMP) receptors and a cytosolic complex termed the inflammasome initiate forms of inflammation. In this study, we examined whether DAMP-receptors and the inflammasome provide the link between cell death and the initiation of inflammation in pancreatitis.
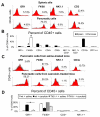
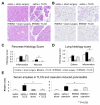
Methods: Acute pancreatitis was induced by caerulein stimulation in wild-type mice and mice deficient in components of the inflammasome (apoptosis-associated speck-like protein containing a caspase recruitment domain [ASC], NLRP3, caspase-1), Toll-like receptor 9 (TLR9), or the purinergic receptor P2X(7). Resident and infiltrating immune cell populations and pro-interleukin-1β expression were characterized in control and caerulein-treated adult murine pancreas. TLR9 expression was quantified in pancreatic cell populations. Additionally, wild-type mice were pretreated with a TLR9 antagonist before induction of acute pancreatitis by caerulein or retrograde bile duct infusion of taurolithocholic acid 3-sulfate.
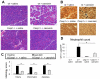
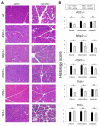
Results: Caspase-1, ASC, and NLRP3 were required for inflammation in acute pancreatitis. Genetic deletion of Tlr9 reduced pancreatic edema, inflammation, and pro-IL-1β expression in pancreatitis. TLR9 was expressed in resident immune cells of the pancreas, which are predominantly macrophages. Pretreatment with the TLR9 antagonist IRS954 reduced pancreatic edema, inflammatory infiltrate, and apoptosis. Pretreatment with IRS954 reduced pancreatic necrosis and lung inflammation in taurolithocholic acid 3-sulfate-induced acute pancreatitis.
Conclusions: Components of the inflammasome, ASC, caspase-1, and NLRP3, are required for the development of inflammation in acute pancreatitis. TLR9 and P2X(7) are important DAMP receptors upstream of inflammasome activation, and their antagonism could provide a new therapeutic strategy for treating acute pancreatitis.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–117. - PubMed
-
- Gravante G, Garcea G, Ong SL, Metcalfe MS, Berry DP, Lloyd DM, Dennison AR. Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology. 2009;9:601–14. - PubMed
-
- Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, Holland S, Pandol SJ. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01DK076674-01A2/DK/NIDDK NIH HHS/United States
- T32 DK007356/DK/NIDDK NIH HHS/United States
- R01 DK076674/DK/NIDDK NIH HHS/United States
- R01 DK083327/DK/NIDDK NIH HHS/United States
- R03DK078707/DK/NIDDK NIH HHS/United States
- K08 DK092281/DK/NIDDK NIH HHS/United States
- K08 DK068116/DK/NIDDK NIH HHS/United States
- R01 DK054021/DK/NIDDK NIH HHS/United States
- R03 DK101847/DK/NIDDK NIH HHS/United States
- P30 DK34989/DK/NIDDK NIH HHS/United States
- K08 DK68116/DK/NIDDK NIH HHS/United States
- R03 DK078707/DK/NIDDK NIH HHS/United States
- DK54021/DK/NIDDK NIH HHS/United States
- T32 DK7356/DK/NIDDK NIH HHS/United States
- P30 DK034989/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

