Exercise-induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation
- PMID: 21439969
- PMCID: PMC3103621
- DOI: 10.1016/j.yjmcc.2011.03.010
Exercise-induced expression of cardiac ATP-sensitive potassium channels promotes action potential shortening and energy conservation
Abstract
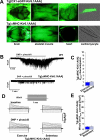
Physical activity is one of the most important determinants of cardiac function. The ability of the heart to increase delivery of oxygen and metabolic fuels relies on an array of adaptive responses necessary to match bodily demand while avoiding exhaustion of cardiac resources. The ATP-sensitive potassium (K(ATP)) channel has the unique ability to adjust cardiac membrane excitability in accordance with ATP and ADP levels, and up-regulation of its expression that occurs in response to exercise could represent a critical element of this adaption. However, the mechanism by which K(ATP) channel expression changes result in a beneficial effect on cardiac excitability and function remains to be established. Here, we demonstrate that an exercise-induced rise in K(ATP) channel expression enhanced the rate and magnitude of action potential shortening in response to heart rate acceleration. This adaptation in membrane excitability promoted significant reduction in cardiac energy consumption under escalating workloads. Genetic disruption of normal K(ATP) channel pore function abolished the exercise-related changes in action potential duration adjustment and caused increased cardiac energy consumption. Thus, an expression-driven enhancement in the K(ATP) channel-dependent membrane response to alterations in cardiac workload represents a previously unrecognized mechanism for adaptation to physical activity and a potential target for cardioprotection.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




References
-
- Guyton AC, Hall JE. Sport physiology. In: Guyton AC, Hall JE, editors. Textbook of medical physiology. Elsevier Saunders; Philadelphia, PA: 2006. pp. 1062–1062-1065.
-
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev. 1999;20:101–35. - PubMed
-
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. - PubMed
-
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

