Features of sepsis caused by pulmonary infection with Francisella tularensis Type A strain
- PMID: 21440052
- PMCID: PMC3090489
- DOI: 10.1016/j.micpath.2011.03.007
Features of sepsis caused by pulmonary infection with Francisella tularensis Type A strain
Abstract
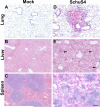
The virulence mechanisms of Francisella tularensis, the causative agent of severe pneumonia in humans and a CDC category A bioterrorism agent, are not fully defined. As sepsis is the leading cause of mortality associated with respiratory infections, we determined whether, in the absence of any known bacterial toxins, a deregulated host response resulting in sepsis syndrome is associated with lethality of respiratory infection with the virulent human Type A strain SchuS4 of F. tularensis. The C57BL/6 mice infected intranasally with a lethal dose of SchuS4 exhibited high bacterial burden in systemic organs and blood indicative of bacteremia. In correlation, infected mice displayed severe tissue pathology and associated cell death in lungs, liver and spleen. Consistent with our studies with murine model strain Francisella novicida, infection with SchuS4 caused an initial delay in upregulation of inflammatory mediators followed by development of severe sepsis characterized by exaggerated cytokine release, upregulation of cardiovascular injury markers and sepsis mediator alarmins S100A9 and HMGB1. This study shows that pulmonary tularemia caused by the Type A strain of F. tularensis results in a deregulated host response leading to severe sepsis and likely represents the major cause of mortality associated with this virulent pathogen.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

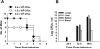

Similar articles
-
Vaccination with an attenuated strain of Francisella novicida prevents T-cell depletion and protects mice infected with the wild-type strain from severe sepsis.Infect Immun. 2009 Oct;77(10):4314-26. doi: 10.1128/IAI.00654-09. Epub 2009 Jul 27. Infect Immun. 2009. PMID: 19635830 Free PMC article.
-
Lethal pulmonary infection with Francisella novicida is associated with severe sepsis.J Leukoc Biol. 2009 Sep;86(3):491-504. doi: 10.1189/jlb.1208728. Epub 2009 Apr 28. J Leukoc Biol. 2009. PMID: 19401387 Free PMC article.
-
MyD88-dependent signaling prolongs survival and reduces bacterial burden during pulmonary infection with virulent Francisella tularensis.Am J Pathol. 2013 Oct;183(4):1223-1232. doi: 10.1016/j.ajpath.2013.06.013. Epub 2013 Aug 3. Am J Pathol. 2013. PMID: 23920326 Free PMC article.
-
Comparative review of Francisella tularensis and Francisella novicida.Front Cell Infect Microbiol. 2014 Mar 13;4:35. doi: 10.3389/fcimb.2014.00035. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24660164 Free PMC article. Review.
-
Live Attenuated Tularemia Vaccines for Protection Against Respiratory Challenge With Virulent F. tularensis subsp. tularensis.Front Cell Infect Microbiol. 2018 May 15;8:154. doi: 10.3389/fcimb.2018.00154. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29868510 Free PMC article. Review.
Cited by
-
Role of NK cells in host defense against pulmonary type A Francisella tularensis infection.Microbes Infect. 2013 Mar;15(3):201-11. doi: 10.1016/j.micinf.2012.11.008. Epub 2012 Dec 1. Microbes Infect. 2013. PMID: 23211929 Free PMC article.
-
The Early Dendritic Cell Signaling Induced by Virulent Francisella tularensis Strain Occurs in Phases and Involves the Activation of Extracellular Signal-Regulated Kinases (ERKs) and p38 In the Later Stage.Mol Cell Proteomics. 2018 Jan;17(1):81-94. doi: 10.1074/mcp.RA117.000160. Epub 2017 Oct 18. Mol Cell Proteomics. 2018. PMID: 29046388 Free PMC article.
-
QTY Code-designed Water-soluble Fc-fusion Cytokine Receptors Bind to their Respective Ligands.QRB Discov. 2020 Apr 9;1:e4. doi: 10.1017/qrd.2020.4. QRB Discov. 2020. PMID: 34192260 Free PMC article.
-
Iron and Virulence in Francisella tularensis.Front Cell Infect Microbiol. 2017 Apr 4;7:107. doi: 10.3389/fcimb.2017.00107. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28421167 Free PMC article. Review.
-
Proteomic analysis of bronchoalveolar lavage fluid proteins from mice infected with Francisella tularensis ssp. novicida.J Proteome Res. 2012 Jul 6;11(7):3690-703. doi: 10.1021/pr3001767. Epub 2012 Jun 22. J Proteome Res. 2012. PMID: 22663564 Free PMC article.
References
-
- Sjostedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci. 2007;1105:1–29. - PubMed
-
- Nano FE, Schmerk C. The Francisella pathogenicity island. Ann N Y Acad Sci. 2007;1105:122–37. - PubMed
-
- Knapp S, Schultz MJ, van der Poll T. Pneumonia models and innate immunity to respiratory bacterial pathogens. Shock. 2005;24(Suppl 1):12–8. - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. - PubMed
-
- Munford RS. Severe sepsis and septic shock: the role of gram-negative bacteremia. Annu Rev Pathol. 2006;1:467–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

