Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells
- PMID: 21440525
- PMCID: PMC3081963
- DOI: 10.1016/j.jinorgbio.2011.02.002
Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells
Abstract
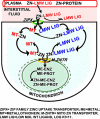
In addition to its critical role in normal cell function, growth, and metabolism, zinc is implicated as a major factor in the development and progression of many pathological conditions and diseases. Despite this importance of zinc, many important factors, processes, and mechanisms of the physiology, biochemistry, and molecular biology of zinc remain unknown. Especially important is the unresolved issue regarding the mechanism and process of the trafficking, transport, and reactivity of zinc in cells; especially in mammalian cells. This presentation focuses on the concept that, due to the existence of a negligible pool of free Zn(2+) ions in the mammalian cell environment, the trafficking, transport and reactivity of zinc occurs via a direct exchange of zinc from donor Zn-ligands to acceptor ligands. This Zn exchange process occurs without the requirement for production of free Zn(2+) ions. The direct evidence from mammalian cell studies is presented in support of the operation of the direct Zn-ligand exchange mechanism. The paper also provides important information and conditions that should be considered and employed in the conduct of studies regarding the role and effects of zinc in biological/biomedical research; and in its clinical interpretation and application.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
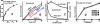






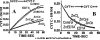


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

